Abstract
Intracellular accumulation of abnormal proteins with conformational changes is the defining neuropathological feature of neurodegenerative diseases. The pathogenic proteins that accumulate in patients' brains adopt an amyloid-like fibrous structure and exhibit various ultrastructural features. The biochemical analysis of pathogenic proteins in sarkosyl-insoluble fractions extracted from patients’ brains also shows disease-specific features. Intriguingly, these ultrastructural and biochemical features are common within the same disease group. These differences among the pathogenic proteins extracted from patients’ brains have important implications for definitive diagnosis of the disease, and also suggest the existence of pathogenic protein strains that contribute to the heterogeneity of pathogenesis in neurodegenerative diseases. Recent experimental evidence has shown that prion-like propagation of these pathogenic proteins from host cells to recipient cells underlies the onset and progression of neurodegenerative diseases. The reproduction of the pathological features that characterize each disease in cellular and animal models of prion-like propagation also implies that the structural differences in the pathogenic proteins are inherited in a prion-like manner. In this review, we summarize the ultrastructural and biochemical features of pathogenic proteins extracted from the brains of patients with neurodegenerative diseases that accumulate abnormal forms of tau, α-synuclein, and TDP-43, and we discuss how these disease-specific properties are maintained in the brain, based on recent experimental insights.
Similar content being viewed by others
Introduction
The pathological hallmark of neurodegenerative diseases is the accumulation of misfolded proteins in neurons and/or glial cells. The accumulation of amyloid-β (Aβ) and tau in the brains of patients with Alzheimer's disease (AD) was reported in the 1980s, and the group of diseases characterized by abnormal tau inclusions is referred to as tauopathies [46, 106, 315]. In 1997 and 1998, it was reported that α-synuclein (α-syn) is a major component of Lewy bodies (LBs) observed in the brains of patients with Parkinson's disease (PD), dementia with Lewy bodies (DLB) and glial cytoplasmic inclusions (GCIs) observed in multiple system atrophy (MSA) [26, 276, 302]. The group of diseases characterized by abnormal α-syn inclusions is termed synucleinopathies. In 2006, TAR DNA-binding protein of 43 kDa (TDP-43) was identified as a major component of the ubiquitin-positive inclusions that accumulate in the brains of patients with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD), and the diseases in which abnormal TDP-43 accumulates are collectively called TDP-43 proteinopathies [14, 216]. These abnormal proteins share common features such as insolubility in detergents, resistance to proteases and formation of amyloid-like fibrous structures, and exhibit prion-like properties, inducing the conversion of normal proteins into an abnormal form [26, 66, 128, 168, 217, 262, 274, 294, 304, 311, 312]. In addition, various post-translational modifications (PTMs), such as phosphorylation and ubiquitination, are detected in detergent-insoluble fractions extracted from patients’ brains [28, 156, 157]. Furthermore, histopathological studies of serial sections of post-mortem brains have shown that the intracerebral accumulation of these abnormal proteins correlates strongly with clinical symptoms and expands in a stereotypic manner [39, 40, 45, 145, 252, 253, 308]. Both the Braak and Murayama groups have proposed staging of the intracerebral expansion of these pathologies, which is widely used to evaluate disease progression [39, 40, 252, 253]. In AD, tau pathology first appears in the locus coeruleus and then expands from the hippocampus to the cerebral cortex through the cerebral limbic system [39, 42]. In argyrophilic grain dementia (AGD), tau pathology develops along the anterior and posterior medial temporal lobe from the ambient gyrus and further extends to the septum, insular cortex and anterior cingulate gyrus [253]. In PD, α-syn pathology spreads from the dorsal motor nucleus of the vagus nerve to the cerebral cortex along the pathway ascending the brainstem [40]. In DLB, the development of α-syn pathology has been reported to start at the olfactory bulb [252]. In ALS, TDP-43 pathology has been reported to spread from the motor cortex or brainstem to the temporal lobe [44, 45]. Furthermore, it has been reported that α-syn pathology was observed in the graft 10–24 years after transplantation of fetal dopamine neurons into PD patients [167, 183, 185]. These reports suggest the possibility that abnormal α-syn was released from the host cells and incorporated into the grafted cells. These pathological findings indicate that abnormal proteins accumulated in the brain self-amplify using themselves as a template, like prions, and spread from cell to cell along the neuronal circuit. This hypothesis is known as prion-like propagation, and self-templated amplification and cell-to-cell transmission of pathogenic tau, α-syn and TDP-43 have been demonstrated in in vitro and in vivo experimental models [66, 162, 199, 217, 236, 242, 314]. These experimental transmissions strongly support the idea that prion-like propagation is involved in the onset and progression of various neurodegenerative diseases. At present, the treatment of neurodegenerative diseases is limited to symptomatic therapy, but the development of novel disease-modifying therapies and agents targeting prion-like propagation is anticipated.
Implications of ultrastructural and biochemical features for the diversity of prion strains
The prion hypothesis, which gave rise to the idea of prion-like propagation, was proposed as a mechanism to explain the pathogenesis of prion diseases by Prusiner [238]. Prion is a term that describes proteinaceous infectious particles that do not contain nucleic acids. Prion protein (PrP), which causes prion diseases such as Creutzfeldt–Jakob disease and bovine spongiform encephalopathy, is rich in α-helices in the normal state (PrPC), whereas when converted to the abnormal form (PrPSc), it forms amyloids rich in β-sheet structures[171, 196, 222]. Then, PrPSc acts as a template and self-amplifies. Amyloid structures known as prion rods are observed in the detergent-insoluble fraction extracted from the brains of patients with prion diseases [32]. This conformational change enables PrPSc to acquire various properties different from those of PrPC. Biochemical analysis of infected animals and post-mortem tissues from patients revealed that PrPSc has unique features that are different from those of viruses, such as resistance to conventional virus inactivation methods including boiling and UV irradiation, insolubility in detergents, and resistance to proteases [206, 218, 239]. Immunoblot analysis of protease-treated PrPSc detects low-molecular-weight protease-resistant bands, and these banding patterns show diversity depending on the type of prion disease and the host species expressing PrPC [139, 166, 224, 231]. Differences in sensitivity to proteases have also been reported [102, 137, 226]. What do these biochemical differences in PrPSc suggest? The diversity in the biochemical properties of PrPSc probably reflects distinct conformations of PrPSc. In PrP “strains" with different conformations, the ultrastructural and biochemical features characterize the strain, and the structural information of PrPSc is crucial for self-amplification. Therefore, the structure of PrPSc may have significant implications for clinical symptoms and pathogenesis. PrP strains have been shown to have different infectivity and incubation periods in experimental animal models [248, 289]. It remains unclear what factors are involved in the formation of different strains from the same protein. However, some cofactors and PTMs may be involved [3, 52]. Recently, it has been demonstrated that strains are also present in amyloidogenic proteins that accumulate in the brains of patients with neurodegenerative diseases other than prion diseases. Among the pathogenic proteins associated with neurodegenerative diseases, tau, α-syn and TDP-43 are intracellularly accumulated and show common PTMs. In addition, there is evidence for a robust link between their accumulation and neurodegeneration [22, 114, 170, 200, 208, 307]. Therefore, we focus here on classification of the ultrastructural and biochemical features of neurodegenerative disease-specific strains of these three proteins.
Ultrastructural and biochemical classification of pathogenic tau derived from human tauopathies
Tau is a microtubule-associated protein localized mainly in axons and is one of the natively unfolded proteins [33, 47, 305]. In the adult human brain, tau exists in six isoforms consisting of 352–441 amino acids, resulting from alternative splicing of exon 2, exons 2 and 3, or exon 10 in the MAPT gene encoding tau [107]. The isoforms with 0, 1 or 2 inserts in the N-terminal repeat domains encoded by exons 2 and 3 are referred to as 0N, 1N and 2N, respectively. Furthermore, these tau isoforms are divided into three-repeat tau (3R tau) and four-repeat tau (4R tau), according to the number of repeats in the microtubule binding domain. The expression ratio of 3R tau to 4R tau is about 1:1 [109]. Under physiological conditions, tau functions to stabilize microtubules and promote their polymerization [67, 68, 141]. Tauopathies are classified into three groups: one in which 3R tau and 4R tau accumulate, one in which only 3R tau accumulates, and one in which only 4R tau accumulates. In AD and chronic traumatic encephalopathy (CTE), all six isoforms of human tau accumulate as neurofibrillary tangles (NFTs) and neuropil threads (Fig. 1a) [27, 113, 244]. In the very early stage of AD, tau-positive deposits with no obvious fibrous structure can be seen with a light microscope, and these are known as pre-tangles [27, 295]. However, electron microscopic studies have revealed fibrous structures in pre-tangles, suggesting that tau filaments in pre-tangles are not densely packed and unbundled, unlike NFTs [27, 288]. The occurrence of pre-tangles probably represents a preliminary stage of NFT formation. The Braak group has assessed pre-tangle stages with AT8-immunopositive tau, in addition to NFT stages with Gallyas silver staining [42]. Astrocytic tangles are also a neuropathological feature in CTE [203]. Primary age-related tauopathy (PART) is characterized by the absence of Aβ accumulation and by NFTs indistinguishable from those of AD [72]. In Pick’s disease (PiD), 3R tau accumulates in neurons as Pick's bodies, which are frequently located in interneurons in layer 2 of the cerebral cortex and the gyrus dentatus (Fig. 1b) [20, 232, 241]. In 4R tauopathies, 4R tau accumulates in neurons and glial cells in various pathological forms. Progressive supranuclear palsy (PSP) is characterized by tufted astrocytes, while corticobasal degeneration (CBD) is characterized by astrocytic plaques (Fig. 1c, d) [92, 134, 316]. In AGD, 4R tau accumulates as pre-tangles in neurons and argyrophilic grains (Fig. 1e) [38, 290]. Globular glial tauopathy (GGT) is pathologically characterized by globular tau-positive inclusions in glial cells [4, 169]. Although the majority of tauopathies are sporadic, more than 50 mutations in the exons and introns of the MAPT gene have been reported to be linked to the onset of tauopathies, and these cases are referred to as frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17T) [103, 143, 235, 275]. It has been reported that missense mutations on the MAPT gene alter the structure of normal tau and promote the formation of tau aggregates [110, 132]. In addition, mutations in the intron region of the MAPT gene affect alternative splicing and alter the expression ratio of 3R tau and 4R tau [120, 300]. These intronic mutations lead to the onset of tauopathies that present clinical symptoms and pathology similar to those of tauopathies in which tau isoforms accumulate (Fig. 1f) [65, 74, 143, 275]. Abnormal tau accumulated in the brain of patients with tauopathies is highly phosphorylated at numerous sites [121, 212]. These abnormal forms of tau lack the ability to bind to microtubules and show various PTMs, such as ubiquitination, acetylation, deamidation and oxidation, as well as phosphorylation [18, 157, 306]. The profiles of these PTMs in AD cases have been shown to change depending on the Braak stage [306]. First, in addition to the phosphorylation observed in the healthy brain, the number of sites and the frequency of phosphorylation in the proline-rich and C-terminal domains increase as the Braak stage progresses [306]. Furthermore, ubiquitination and acetylation in the microtubule-binding domain become more prominent at later Braak stages, suggesting that these PTMs may act as a degradation signal after NFTs are formed [306]. In addition to the PTMs common to tauopathies, disease-specific PTMs have been reported [157]. Non-phosphorylation of S356 and deamidation of N279 are distinctive features of insoluble tau extracted from AD cases [77, 157]. PiD is the only tauopathy in which S262 is not phosphorylated [16]. Multiple disease-specific ubiquitination (K343, K353, K369 and K375) is detected in CBD, whereas PSP is characterized by less ubiquitination [157]. Unlike other tauopathies, tau deposits in AGD cases have been reported to lack acetylation of K279 [119]. The abnormal tau is also known to be increased not only in the brain, but also in cerebrospinal fluid (CSF) and blood of patients with tauopathies [75, 151, 201, 299]. It has been reported that the influx of abnormal tau into CSF and blood may be a result of aging-related disruption of the blood–brain barrier, and that specific phosphorylation sites of tau in CSF and plasma may be useful as biomarkers to identify the early stage of tauopathies [149, 210, 278]. Neurofilament proteins and heparansulfate proteoglycans are known to show co-immunoreactivity in tau pathology, and neurofilament light chain is used as a biomarker of neurodegeneration [11, 220, 229, 271]. Heparansulfate proteoglycans have been reported to be involved in cell-to-cell transmission of pathogenic tau [140, 161].
Ultrastructural and biochemical characterization of pathogenic tau extracted from human tauopathies. Immunohistochemistry of brain section from patients with tauopathies, stained with AT8 antibody. a Neurofibrillary tangles and neuropil threads in AD. b Pick bodies in PiD. c Tufted astrocyte in PSP. d Astrocytic plaque in CBD. e Neuronal inclusions and glial inclusions in FTDP-17T (+ 16). f Argyrophilic grains in AGD. Scale bar, 50 μm. g Immunoelectron microscopy of sarkosyl-insoluble fractions extracted from brains of tauopathy patients. Electron micrographs show fibrous structures positive for anti-tauC, after labeling with secondary antibody conjugated to 5 nm gold particles. Paired helical filaments (PHF, 10–20 nm in diameter) and straight filaments (SF, 15 nm in diameter) in AD, straight filaments (13–17 nm in diameter) in PiD, twisted ribbon-like filaments (15 nm in diameter) in PSP, twisted filaments (10–30 nm in diameter) in CBD, twisted filaments (7–25 nm in diameter) in FTDP-17T (+ 14, + 16), twisted ribbon-like filaments in GGT, and twisted filaments in AGD were observed. Scale bar, 50 nm. h Immunoblot analyses of sarkosyl-insoluble fractions prepared from brains of tauopathy patients. Sarkosyl-insoluble full-length tau (60, 64 and 68 kDa) and C-terminal fragments were detected with T46 antibody (residues 404–441). Disease-specific C-terminal fragments were also detected: 19, 22, 25, 30, 36 and 40 kDa bands in AD, 21, 34 and 39 kDa bands in PiD, 22 and 33 kDa bands in PSP and GGT, 22, 37 doublet and 43 kDa bands in CBD, FTDP-17T (+ 14, + 16) and AGD. All data are original for this review
In the brain of patients with tauopathies, abnormal tau accumulates as amyloid-like filaments rich in β-sheets. In 1963, the fibrous structure in the brain of AD patients was first reported on the basis of an electron microscopic study [163]. Furthermore, it has been revealed that this twisted fibrous structure is tau filaments composed of two protofilaments, known as paired helical filaments (PHFs), and that PHFs and straight filaments (SFs) are observed in the brains of AD patients [73, 112, 180]. Electron microscopy of the sarkosyl-insoluble fraction extracted from AD cases shows that PHFs are 10–20 nm in diameter and twisted with 80 nm periodicity, while SFs are 15 nm in diameter (Fig. 1g). In other tauopathies, abnormal tau also forms various disease-specific fibrous structures [19, 108, 284]. Tau filaments extracted from PiD cases are 13–17 nm in diameter and straight or twisted (Fig. 1g). Tau filaments 15 nm in diameter from PSP cases are loosely twisted ribbons, while those from CBD cases are 10–30 nm in diameter and twisted with 140 nm periodicity (Fig. 1g). In FTDP-17T (intron 10 mutations + 14 and + 16), tau filaments 7–25 nm in diameter are twisted with 240 nm periodicity, and these structures are different from those of filaments extracted from PSP and CBD cases (Fig. 1g). Tau filaments from GGT are ribbon-like, while those from AGD are twisted with a relatively long periodicity (Fig. 1g). In recent years, cryogenic electron microscopy (cryo-EM) analysis of sarkosyl-insoluble fractions extracted from the brains of patients with tauopathies has revealed the core structures of tau filaments that characterize each disease [266]. A C-shaped core structure consisting of V306-F378 in 3R tau and G304-E380 in 4R tau was identified in the brain of one individual with AD (Table 1) [98]. Although the protofilaments in PHFs and SFs share a common fold, the interfaces of the protofilaments are different (Table 1) [98]. The AD fold was also identified in insoluble fractions extracted from multiple AD cases, and the structures of tau filaments extracted from a familial AD case with a V717F mutation in the APP gene, which produces Aβ, were identical to those of sporadic AD cases [90]. Tau filament structures identical with those in sporadic AD cases have also been found in other tauopathies including PART, posterior cortical atrophy (PCA), familial British dementia (FBD) and familial Danish dementia (FDD) and in prion diseases including PrP cerebral amyloid angiopathy (PrP-CAA) and Gerstmann-Sträussler-Scheinker disease (GSS) [127, 265, 266]. In CTE cases, a fibrous core structure consisting of K274-R379 in 3R tau and S305-R379 in 4R tau was identified, and the CTE fold was different from the AD fold (Table 1) [91]. A nonproteinaceous molecule was also present within the CTE fold (Table 1) [91]. The J-shaped core structure of tau filaments extracted from PiD cases involves K254-F378 in 3R tau, and although most of the filaments were of single protofilament type, twisted filaments composed of two protofilaments were also identified (Table 1) [89]. Tau filaments extracted from CBD cases consist of single protofilament and doublet types, and a CBD fold consisting of K274-E380 in 4R tau, having a nonproteinaceous molecule within the fold, was identified (Table 1) [322]. It has been reported that the pre-tangles observed in CBD cases show a different fibrous morphology from those of AD cases, which may be due to differences in the stability and the interaction between the two protofilaments of AD and CBD filaments [21, 282, 288]. The PSP fold, which is structurally different from the CBD fold, consists of G272-N381 in 4R tau and is identical in typical and atypical PSP cases (Table 1) [266]. In GGT cases, a fibrous core structure consisting of G272-R379 in 4R tau, akin to the PSP fold, was identified, and while only protofilament-type tau filaments were found in PSP cases, multiple structures consisting of two protofilaments packed at different interfaces were found in type I and type II cases in the three types of GGT (Table 1) [266]. In AGD, FTDP-17T (intron 10 mutations + 3 and + 16) and aging-related tau astrogliopathy (ARTAG) cases, a fibrous core structure akin to the CBD fold, consisting of G273-D387 or N279-N381 in 4R tau, was identified (Table 1) [266]. Intriguingly, the disease-specific PTMs in the tauopathies described above are detected in and around these fibrous core structures [157]. The non-phosphorylation at K356 in AD cases and S262 in PiD cases is explained by the fact that these residues are in locations that are not accessible from the outside in each fold, suggesting that phosphorylation at these residues may be able to occur after the fold formation in tauopathies [89, 98]. These disease-specific tau fibrous core structures and the correspondence of these structures within the same disease group provide a direct demonstration of tau strain formation in the patient’s brain. Although the nonproteinaceous molecules within the CTE fold and the CBD fold have not been identified, these molecules may cause the structural differences from the AD fold and the AGD/FTDP-17T/ARTAG fold, respectively. Recombinant tau purified from E. coli also forms amyloid-like filaments akin to patient-derived tau filaments in the presence of polyanions such as heparin and dextran sulfate [111, 129]. Structural analysis of these synthetic tau filaments by cryo-EM revealed one type of fibrous core structure from synthetic 2N3R tau filaments and four types of fibrous core structure from synthetic 2N4R tau filaments [321]. However, these fibrous core structures are not consistent with any of those derived from patients’ brains. This difference may be attributed to the effect of polyanions, which are essential for the formation of synthetic tau filaments. On the other hand, it has been reported that a synthetic peptide consisting of tau I297-E391 forms PHF-like filaments without polyanions [5, 6]. It has also been suggested that some cell-derived factors and cofactors influence tau filament formation, and that the nonproteinaceous molecules in the CTE fold and the CBD fold may originate from the glial cell environment [97, 309].
Consistent with the structural diversity of tau filaments, the banding patterns of abnormal tau shown by immunoblotting of sarkosyl-insoluble fractions extracted from the brains of patients biochemically characterize tauopathies [15, 284]. Six isoforms of full-length tau are presented as 60, 64, and 68 kDa bands in AD cases, and three isoforms of full-length 3R tau are detected as 60 and 64 kDa bands in PiD cases (Fig. 1h). In 4R tauopathies, including PSP, CBD, FTDP-17T (+ 14 and + 16), GGT, and AGD cases, three isoforms of full-length 4R tau are detected as 64 and 68 kDa bands (Fig. 1h). Tau C-terminal fragments (CTFs) detected by antibodies that recognize the C-terminal of tau also characterize tauopathies. CTFs of 19, 22, 25, 30, 36, and 40 kDa are detected in AD cases, and 21, 34, and 39 kDa in PiD cases (Fig. 1h). CTFs resembling those in AD cases have been reported in CTE cases [219]. In PSP and CBD cases, different CTFs of 22, 33 kDa and 22, 37 doublet, 43 kDa, respectively, are detected (Fig. 1h). These CTF banding patterns are common within the same disease group. The CTF banding patterns of other 4R tauopathies can be divided into PSP-type and CBD-type. CTFs of insoluble tau extracted from GGT cases are PSP-type, whereas CTFs of insoluble tau extracted from FTDP-17T (+ 14, + 16) and AGD cases are CBD-type (Fig. 1h). These disease-specific CTF banding patterns may result from differences in the processing of full-length tau after filament formation, and also reflect the similarity of structural features of tau filaments, as demonstrated by the above cryo-EM structural analyses. The PSP and GGT folds show similar three-layered structures, whereas the CBD and AGD/FTDP-17T folds show similar four-layered structures (Table 1). The trypsin-treated insoluble tau derived from patients’ brains also displays trypsin-resistant bands specific to each tauopathy [284]. Mass analysis of these trypsin-treated insoluble tau revealed disease-specific trypsin-resistant core sequences [284]. These sequences match the sequences of the fibrous core structure revealed by cryo-EM analysis.
Experimental prion-like amplification and transmission of patient-derived tau strains
Insoluble tau extracted from a patient’s brain acts as seeds in vitro, in cultured cells, in primary cultures, and in animal brains, and induces seed-dependent tau aggregation. In in vitro systems such as protein misfolding cyclic amplification (PMCA) and real-time quaking-induced conversion (RT-QuIC), recombinant tau purified from E. coli and samples derived from patients with tauopathies are mixed and incubated with thioflavin, a ligand that specifically binds to β-sheet structure, with shaking or sonication [24, 59]. Seed-dependent tau aggregation is monitored in terms of thioflavin fluorescence intensity. It has been reported that RT-QuIC using brain homogenates can distinguish AD and CTE cases from PiD cases based on the reactivity with thioflavin T [172, 249]. Similarly, brain homogenates and CSFs from PSP and CBD cases can be diagnosed with RT-QuIC [250]. In addition, introduction of patient-derived tau seeds into cultured cells or primary cultures expressing tau induces intracellular tau aggregation [213, 287, 313]. Insoluble tau extracted from AD cases (AD-tau) recruits both 3R tau and 4R tau for aggregation, whereas insoluble tau extracted from PiD cases (PiD-tau) or 4R tauopathy cases causes aggregation of only 3R tau or 4R tau, respectively. Furthermore, it has been reported that the introduction of patient-derived tau seeds into fluorescence resonance energy transfer biosensor cells induces the formation of tau aggregates with various morphologies, and that these morphological differences are inherited during the passaging of cells [162]. Inoculation of patient-derived tau seeds into mouse brain also leads to the formation of tau pathology associated with the inoculated sample. Brain homogenates derived from AD, PSP, CBD and AGD cases induced inoculated-sample-associated tau pathology in human tau transgenic (Tg) mouse brain, whereas the brain homogenates derived from PiD cases did not induce Pick body-like tau pathology [66]. Tau pathologies associated with inoculated samples, including the cell type specificity, are also observed after the inoculation of wild-type mice with brain homogenates or insoluble fractions extracted from tauopathy cases [125, 213]. Furthermore, it has been reported that disease-associated tau pathology is formed in mice expressing all six isoforms of human tau [136]. AD-tau induced tau pathology involving all the tau isoforms, while PiD-tau and insoluble tau extracted from PSP cases (PSP-tau) and CBD cases (CBD-tau) induced tau pathology composed only of 3R tau or 4R tau, respectively [136]. These results suggest that the conformation of tau filaments accumulated in the brains of patients with tauopathies is involved in the formation of the tau pathologies that characterize each disease.
Ultrastructural and biochemical classification of pathogenic α-synuclein derived from human synucleinopathies
α-Synuclein is a synaptic protein that is abundant in the brain and localizes to presynaptic terminals [148, 198]. It consists of 140 amino acids in three domains, the N-terminal domain, the hydrophobic region known as the NAC (non-amyloid core), and the C-terminal proline-rich hydrophobic region [296, 318]. Under physiological conditions, α-syn is a water-soluble, natively unfolded protein, and its function remains unclear [194]. However, the phenotype of α-syn-knockout mice has led to the suggestion that α-syn may be involved in the release of dopamine [1, 61]. It has also been shown that α-syn is involved in the regulation of SNARE complex formation, suggesting that it may act as a molecular chaperone [53, 62]. Synucleinopathies are classified into two major groups, Lewy body disease (LBD), which is characterized by α-syn inclusions in neurons, and MSA, which is characterized by α-syn inclusions mainly in oligodendrocytes. In LBD including PD, Parkinson's disease with dementia (PDD), and DLB, α-syn accumulates in neuronal cell bodies and neurites as LBs and Lewy neurites (LNs) (Fig. 2a) [276]. Diffuse α-syn-immunopositive deposits and pale bodies with fibrous structures are observed in neuronal cells of LBD cases, suggesting that they are formed in the preliminary stages of LB formation [76, 115, 301]. In addition to the Braak staging, the McKeith type is also widely used as a neuropathological staging system of LBD [40, 205]. Braak staging assesses the expansion of Lewy pathology, whereas the McKeith type classifies LBD into five neuropathological subtypes according to the severity of LB pathology in the anatomical regions [40, 204]. MSA is categorized into MSA with predominant parkinsonian features (MSA-P) and MSA with predominant cerebellar ataxia (MSA-C), and in both cases, α-syn accumulates in oligodendrocytes as GCIs (Fig. 2b) [105, 223, 302]. Abnormal α-syn accumulated in the brain of patients with synucleinopathies is phosphorylated at S129 and also partially ubiquitinated [9, 101, 130]. More than 90% of α-syn is phosphorylated in DLB patients’ brains, whereas only 4% is phosphorylated in healthy brains [101]. In LBD patients, phosphorylated α-syn pathology is detected not only in the brain, but also in peripheral tissues such as esophagus, heart, skin, adrenal gland, gut, and sympathetic ganglion [30, 283]. α-Syn is expressed in various peripheral tissues, and the spreading of α-syn pathology from peripheral tissues to the central nervous system has also been proposed [41, 135, 296]. In addition, it has been reported that the concentration of α-syn is increased in CSF and blood derived from patients with synucleinopathies, and this finding is expected to lead to a practical method for early diagnosis [34, 85, 257]. However, since α-syn is abundantly present in platelets, diagnosis using blood samples would need to be performed carefully [133]. Nine missense mutations (A30P, A30G, E46K, H50Q, G51D, A53T, A53E, A53V and E83Q) in the SNCA gene encoding α-syn have been reported to cause familial synucleinopathies [13, 159, 173, 182, 225, 233, 237, 319, 320]. These mutations have been experimentally found to exhibit differences in cytotoxicity, association with cell membranes and lipids, and filament formation as compared with wild-type [64, 84, 104, 153, 209, 247, 255]. Duplication and triplication of the SNCA gene also cause early-onset LBD [63, 94, 144, 268]. It has been suggested that PrPC and lymphocyte-activation gene 3 (LAG3) bind to α-syn filaments and participate in cell-to-cell transmission of pathogenic α-syn as a receptor, but this remains controversial, with several reports suggesting non-binding of PrPC to α-syn and lack of LAG3 expression in human neurons [71, 87, 175, 197, 297].
Ultrastructural and biochemical characterization of pathogenic α-syn extracted from human synucleinopathies. Immunohistochemistry of brain section from patients with synucleinopathies, stained with pS129 antibody. a Lewy bodies and Lewy neurites in DLB. b Glial cytoplasmic inclusions in MSA. Scale bar, 50 μm. c Immunoelectron microscopy of sarkosyl-insoluble fractions extracted from brains of synucleinopathy patients. Electron micrographs show fibrous structures with 5–10 nm in diameter positive for pS129, after labeling with secondary antibody conjugated to 5 nm gold particles. Straight filaments in LBD (PDD and DLB), and twisted filaments with 80–100 nm periodicity in MSA were observed. Scale bar, 50 nm. d Immunoblot analyses of sarkosyl-insoluble fractions prepared from brains of synucleinopathy patients. Sarkosyl-insoluble phosphorylated α-syn (17 kDa) was detected with pS129 antibody. Disease-specific high-molecular-weight α-syn species were also observed: 22, 29, and 37 kDa bands in LBD (PDD and DLB), and 22 and 32 kDa bands in MSA. All data are original for this review
In the brains of patients with synucleinopathies, abnormal α-syn accumulates in the form of amyloid-like fibrous structures [273, 274, 286]. Fibrous structures in LBD brains were first reported in the 1960s [83, 246]. Electron microscopy of the sarkosyl-insoluble fraction extracted from the brains of patients with synucleinopathies shows α-syn filaments 5–10 nm in diameter, and there is a structural difference between α-syn filaments derived from LBD and MSA cases (Fig. 2c). α-Syn filaments extracted from LBD cases are straight, whereas those extracted from MSA cases are twisted with 80–100 nm periodicity (Fig. 2c). In addition, α-syn filaments derived from LBD cases are thinner than α-syn filaments derived from MSA cases (Fig. 2c). The core structures of α-syn filaments from MSA cases, including both MSA-C and MSA-P, have been identified by cryo-EM analysis [258]. Two types of α-syn filaments, type I and type II, have been identified [258]. Type I filaments comprise two protofilaments, PF-IA consisting of G14-F94 and PF-IB consisting of K21-Q99, while type II filaments comprise two protofilaments, PF-IIA consisting of G14-F94 and PF-IIB consisting of G36-Q99 (Table 1) [258]. Furthermore, additional densities between the two protofilaments, not due to α-syn, was seen in both type I and type II (Table 1) [258]. Although the details of these putative nonproteinaceous molecules remains unclear, it is suggested that they are involved in the assembly of the two protofilaments. Two-dimensional analysis showed that α-syn filaments extracted from DLB cases are not twisted, unlike those extracted from MSA cases [258]. The core structures of wild-type and familial synucleinopathy-related mutant-type synthetic α-syn filaments have also been reported [36, 37, 122, 186, 279, 280, 323]. As in the case of synthetic tau filaments, none of the structures of synthetic α-syn filaments are consistent with the structures of type I and type II filaments identified from MSA cases.
Abnormal α-syn accumulated in the brains of patients with synucleinopathies is detected by immunoblotting as a 17 kDa band (Fig. 2d) [286]. The high-molecular-weight bands detected by α-syn antibodies are different between LBD and MSA: 22, 29, and 37 kDa bands are detected in LBD, while 22 and 32 kDa bands are detected in MSA (Fig. 2d) [286]. It has been reported that each of the three high-molecular-weight bands observed in DLB is ubiquitinated α-syn, suggesting that the PTM patterns of abnormal α-syn are different in LBD and MSA [9]. The sensitivity to protease and the protease-resistant banding patterns of insoluble α-syn extracted from LBD and MSA cases are also different [228]. Insoluble α-syn extracted from MSA cases (MSA-syn) is more resistant to protease K and pronase than insoluble α-syn from LBD cases (LBD-syn) [228]. Furthermore, MSA-syn has high SDS solubility, while LBD-syn shows low SDS solubility [55].
Experimental prion-like amplification and transmission of patient-derived α-synuclein strains
Like tau, insoluble α-syn extracted from the brain of patients with synucleinopathies exhibits prion-like properties and causes seed-dependent α-syn aggregation in vitro and in vivo. Heterogeneity of the prion-like seeding activity has also been reported, depending on the α-syn strain [228, 286, 314]. Similar to the case of tauopathy, abnormal α-syn in brain, CSF and skin samples derived from patients with synucleinopathies have been shown to be specifically detected by PMCA and RT-QuIC methods and to be useful biomarkers in the early stages of synucleinopathies [88, 174, 245, 256, 264]. PMCA analysis using brain samples and CSF derived from synucleinopathy cases has revealed differences in the seeding activity of α-syn derived from PD cases (PD-syn) and MSA-syn [263]. MSA-syn induced a rapid increase in thioflavin fluorescence intensity compared to PD-syn, while the fluorescence value at the plateau when PD-syn was added was much higher than when MSA-syn was added [263]. The variation in fluorescence values at the plateau may reflect the structural difference between PD-syn and MSA-syn in terms of the binding mode of α-syn filaments to thioflavin. The α-syn aggregates amplified by PMCA from PD cases showed four bands of 4–10 kDa after protease K treatment, whereas only two bands of 4 and 6 kDa were detected in the case of α-syn aggregates derived from MSA cases [263]. These two types of protease K-resistant banding patterns suggest that distinct PMCA products are formed depending on the template. The difference in seeding activity between LBD-syn and MSA-syn (i.e., MSA-syn induces greater intracellular α-syn aggregation than LBD-syn) is also observed in cultured cells and primary cultures [228, 286, 314, 317]. Furthermore, the potent prion-like character of MSA-syn is manifested in the brains of α-syn A53T Tg mice and wild-type mice [228, 240, 286, 304]. However, in mouse brains inoculated with MSA-syn, α-syn pathology is observed in neurons, but not in oligodendrocytes [178, 286, 304]. This may be explained by the level of α-syn expression in oligodendrocytes. In situ hybridization analyses of healthy brains and MSA brains indicate that α-syn expression is very low in oligodendrocytes [152, 207, 272]. It is possible that normal or oligomeric α-syn before the formation of β-sheet structure is secreted from neurons and incorporated into oligodendrocytes. α-Syn pathology in oligodendrocytes has been shown to form in Tg mice that express α-syn in oligodendrocytes [228]. Further studies are required to clarify the involvement of neuron-derived α-syn in the GCI formation in MSA. In addition, the cellular environment of oligodendrocytes, which is different from that of neurons, may affect the formation of α-syn filaments, resulting in the generation of α-syn strains. The possibility that different cellular environments may be involved in the formation of distinct α-syn strains has been suggested based on studies using recombinant α-syn. The synthetic α-syn filaments formed under various physiological conditions exhibit distinct morphological and physical properties, and exhibit different seeding activity, cytotoxicity and proteosome activity in vitro, in cultured cells and in primary cultures [35, 118, 281]. Synthetic α-syn filaments formed in the presence of physiological salt concentration showed significant cytotoxicity, while those formed in the absence of salt showed high seeding activity in rat brain [227]. Intracerebral inoculation of these synthetic α-syn strains also causes various α-syn pathological morphologies and propagation patterns in the brains of α-syn A53T Tg mice and wild-type mice [177, 281].
Ultrastructural and biochemical classification of pathogenic TDP-43 derived from human TDP-43 proteinopathies
TDP-43 is an RNA-binding nuclear protein localized mainly in the nucleus [25]. It consists of 411 amino acids with a nuclear translocation signal (NLS) sequence, and is composed of the N-terminal domain, two RNA recognition motif domains, and the C-terminal glycine-rich domain [48, 221]. The physiological functions of TDP-43 include the regulation of processing functions such as pre-mRNA splicing, and it is involved in the transport of mRNA from the nucleus to the cytoplasm [7, 48, 49, 69, 291]. TDP-43 was reported to be a major component of the ubiquitin-positive inclusion bodies observed in the brains of patients with FTLD and ALS [14, 216]. The abnormal TDP-43 inclusions mostly accumulate in the cytoplasm, and the nuclear localization of TDP-43 and its physiological functions are lost [50, 310]. FTLD-TDP, in which phosphorylated and ubiquitinated TDP-43 accumulates, is classified into five subtypes (type A-E) based on differences in pathology and distribution [179, 193]. Type A is defined by neuronal cytoplasmic inclusions (NCIs) and short dystrophic neurites (DNs) (Fig. 3a) [193]. Type B, which includes ALS and FTLD with motor neuron disease, presents predominantly NCI pathology (Fig. 3b) [193]. Type C is characterized by long DNs (Fig. 3c) [193]. Type D presents predominantly intranuclear inclusion pathology [192, 193]. Type E is characterized by granulofilamentous neuronal inclusions and very fine, dot-like neuropil inclusions [179]. TDP-43-immunopositive diffuse or granular or dash-like cytoplasmic inclusions are observed in the brains of FTLD-TDP patients, and the formation of these pre-inclusions may represent the early stage of pathogenesis in TDP-43 proteinopathy [43, 78, 211]. Limbic-predominant age-related TDP-43 encephalopathy (LATE) is neuropathologically characterized by the accumulation of phosphorylated TDP-43 in the limbic system and leads to AD-like symptoms in elderly individuals [214]. Missense mutations on the TARDBP gene encoding TDP-43 are associated with the onset of ALS and FTLD-TDP [160]. More than 30 mutations have been reported, and most of them are located in the C-terminal low-complexity domain [155, 277]. Mutations in other RNA-binding proteins, including hnRNPA1, hnPA2B1 and MATR3, and a hexanucleotide repeat expansion in the C9ORF72 gene also cause TDP-43 proteinopathy, suggesting that the interaction of these RNA-binding proteins with TDP-43 and disruption of RNA metabolic homeostasis are involved in the intracytoplasmic accumulation of TDP-43 [80, 154, 164, 243]. Recently, the association between the formation of stress granules and the accumulation of TDP-43 in the cytoplasm has been investigated [10, 81]. Under various stress conditions, such as oxidative stress and heat shock, stress granules, which are membrane-less organelles composed of RNA and RNA-binding proteins, are formed via liquid–liquid phase separation, and it has been reported that they take up endogenous TDP-43 [70, 95, 187]. However, it is not clear whether stress granule formation directly leads to TDP-43 pathogenesis, and further studies are required.
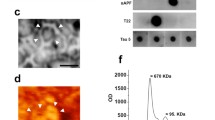
Ultrastructural and biochemical characterization of pathogenic TDP-43 extracted from human TDP-43 proteinopathies. Immunohistochemistry of brain section from patients with TDP-43 proteinopathies, stained with pS409/pS410 antibody. a Neuronal cytoplasmic inclusions (NCIs) and short degenerative neurites (DNs) in FTLD-TDP Type A. b NCIs in FTLD-TDP Type B. c Long DNs in FTLD-TDP Type C. Scale bar, 50 μm. d Immunoelectron microscopy of sarkosyl-insoluble fractions extracted from FTLD-TDP (type A, type B, type C) patients. Electron micrographs show fibrous structures with 10–15 nm in diameter positive for pS409/pS410, after labeling with secondary antibody conjugated to 5 nm gold particles. Scale bar, 50 nm. e Immunoblot analyses of sarkosyl-insoluble fractions prepared from brains of patients withTDP-43 proteinopathies. Sarkosyl-insoluble phosphorylated TDP-43 (45 kDa) was detected with pS409/410 antibody. Subtype-specific C-terminal fragments were also observed: 18, 19, 23, 24 and 26 kDa bands in type A, 18, 19, 23, 24 and 26 kDa bands in type B, 18, 19, 23 and 24 kDa bands in type C. All data are original for this review
Immuno-electron microscopic studies of brain sections from cases of ALS and FTLD-with ubiquitin-positive inclusions have shown the presence of TDP-43 and ubiquitin-positive bundles of straight filaments 10–20 nm in diameter in neurons [147, 165, 188]. Amyloid-like filaments 10–15 nm in diameter are also observed by electron microscopy of insoluble fractions extracted from the brains of patients with TDP-43 proteinopathy (Fig. 3d) [128, 217]. The ultrastructural features of TDP-43 filaments derived from types A-C are distinct for each subtype, and TDP-43 filaments derived from type B are thinner than those derived from other subtypes (Fig. 3d). Synthetic peptides G274-F313 and G314-N353 of TDP-43 were reported to form filaments in vitro, and these filaments possess seeding activity that recruits wild-type TDP-43 or TDP-43 lacking NLS expressed in cultured cells for aggregation, suggesting that residues 274–353 are important for TDP-43 filament formation [267]. Furthermore, cryo-EM analysis of TDP-43 filaments formed from SegA (residues 311–360) and SegB (residues 286–331 including the ALS hereditary mutation A315E) peptides has revealed three different dagger-shaped core structures from SegA filaments and one core structure composed of four R-shaped folds from SegB filaments [56]. In addition, a fibrous core structure consisting of F276-M414 has been identified from TDP-43 filaments composed of the low-complexity domain (residues 267–414) formed under a mildly acidic condition (pH 4) [184]. Cryo-EM analysis of TDP-43 filaments extracted from two individuals diagnosed as ALS with FTLD identified a double-spiral-shaped fold consisting of G282-Q360, which is different from the fibrous core structures of the three synthetic TDP-43 filaments described above (Table 1) [23]. The structure of TDP-43 filaments extracted from brains of patients with other subtypes of FTLD-TDP should also be amenable to cryo-EM analysis. Immunoblotting with phosphorylated S409/S410 antibody of sarkosyl-insoluble fractions extracted from the brains of patients with TDP-43 proteinopathy shows a 45 kDa band of phosphorylated full-length TDP-43 and subtype-specific CTFs bands (Fig. 3e) [128, 131]. In type A, major bands of 23, 24, and 26 kDa and minor bands of 18 and 19 kDa are present and the 23 kDa band is the most intense (Fig. 3e). Similarly, CTFs of 18, 19, 23, 24, and 26 kDa, with the most intense band at 24 kDa, are seen in type B (Fig. 3e). In type C, major bands of 23 and 24 kDa, of which the 23 kDa band is more intense, and minor bands of 18 and 19 kDa are seen (Fig. 3e). These banding patterns of CTFs are common within the same subtype group and may reflect differences in the N-terminal truncation of full-length TDP-43 among the subtypes [294]. A single CTF banding pattern was detected even in the insoluble fractions extracted from different brain regions and spinal cord of one individual [294].It has been reported that the protease-resistant banding patterns of patient-derived insoluble TDP-43 are also common within the same subtype group, but are different between subtypes [294]. In addition, the major PTMs detected in insoluble fractions extracted from ALS cases are phosphorylation, oxidation and deamidation, and partial ubiquitination is also detected [156]. These PTMs are mainly located in the C-terminal domain [156]. While phosphorylation at S403/S404 and at S409/S410 is a definitive pathological marker for all subtypes, differences in immunoreactivity to phosphorylation at S369 have been reported: TDP-43 pathology in type B and type C is pS369-positive, whereas type A is pS369-negative [128, 215]. As with tau and α-syn, these ultrastructural and biochemical variations may reflect differences in the conformation of abnormal TDP-43 accumulated in the brain, suggesting the formation of TDP-43 strains in TDP-43 proteinopathies. The ultrastructural and biochemical features of pathogenic TDP-43 that accumulate in other subtypes and LATE require further investigation.
Experimental prion-like amplification and transmission of patient-derived TDP-43 strains
Patient-derived insoluble TDP-43 exhibits prion-like properties in vitro and in vivo. An RT-QuIC study found that abnormal TDP-43 in brain homogenates and CSF derived from ALS and FTLD cases were amplified using full-length TDP-43 and truncated TDP-43 (residues 263–414) purified from E. coli as substrates [259]. Abnormal TDP-43 could be used as a biomarker for early diagnosis. Introduction of insoluble TDP-43 extracted from the brains of patients with FTLD-TDP types A-C into cultured cells expressing wild-type TDP-43 or TDP-43 lacking NLS causes seed-dependent accumulation of phosphorylated TDP-43 [217]. Furthermore, the insoluble fractions extracted from the cells into which patient-derived TDP-43 seeds had been introduced showed CTF banding patterns similar to those of the patient-derived original seeds [217]. Insoluble TDP-43 extracted from FTLD-TDP type A-C cases by Sarkospin also shows differences of cytotoxicity and seeding activity in cultured cells depending on the subtype [79, 176]. In addition, it has been reported that insoluble TDP-43 extracted from ALS cases induces the formation of phosphorylated TDP-43 inclusions with various morphologies, as observed in the brain of ALS patients in cultured cells expressing wild-type TDP-43, and that the prion-like property of insoluble TDP-43 accumulated in cultured cells is inherited after passaging of the cells [269]. In vivo, intracerebral inoculation of the insoluble fractions extracted from the brains of patients with sporadic FTLD-TDP and familial FTLD-TDP with the GRN or C9ORF72 gene mutations into Tg mice expressing human TDP-43 in the cytoplasm reproduced TDP-43 pathology associated with the inoculated samples in a time-dependent manner [236]. Inoculation of these patient-derived samples into wild-type mice also induced TDP-43 pathology, although the pathology was not as abundant as in the case of inoculation into Tg mice [236].
How are the characteristic structural and biochemical properties of each disease maintained in the patient's brain?
As we have described, abnormal proteins that accumulate in the brains of patients exhibit ultrastructural and biochemical properties that are characteristic of each disease. It is worth emphasizing that these properties are common in the same disease group, and even in different brain regions of one individual. These results suggest that pathogenic tau, α-syn and TDP-43 spread throughout the brain, retaining their disease-specific structural and biochemical properties.
To further explore this possibility, we examined whether disease-specific tau aggregation and filament formation were reproduced in cultured cells [287]. Insoluble tau extracted from the brains of patients with tauopathies was introduced into SH-SY5Y cells expressing full-length 3R tau or 4R tau. PiD-tau induced only 3R tau aggregation, while PSP-tau and CBD-tau induced only 4R tau aggregation. AD-tau recruited both 3R tau and 4R tau for aggregation. This strain-specific tau aggregation was also observed in cells expressing both 3R tau and 4R tau. In addition, immunoelectron microscopy of insoluble tau accumulated in SH-SY5Y cells showed abundant tau filaments labeled by antibody to the tag protein expressed in the cells, and their structures resembled those of filaments derived from the patients’ brains. This template-dependent amplification of tau filaments observed in cultured cells can be explained in terms of the core structure of tau filaments extracted from the brain. Since the AD fold is composed of the common part of 3R tau and 4R tau, AD-tau can recruit both 3R tau and 4R tau for seed-dependent aggregation (Fig. 4). On the other hand, the PiD fold, the PSP fold and the CBD fold contain 3R tau- or 4R tau-specific sequences and therefore, polymerization of β-sheet structure is not promoted when there is a mismatch between template and substrate (Fig. 4). In other words, the structure of the tau filaments determines which tau isoform is recruited for seed-dependent aggregation, and is the determinant of the formation and spreading of tau pathology in the brain. Trypsin-treated patient-derived tau seeds, which were digested outside the tau fibrous core region (fuzzy coat), caused the same seed-dependent aggregation as untreated tau seeds, supporting the idea that the fibrous core region plays a cardinal role in the amplification of tau filaments. Thus, our results suggest that the template-dependent amplification of pathognomonic proteins in the brain leads to the pathological diversity in the same proteinopathy.
Full matching of amino acid sequence between template and substrate is essential for template-dependent amplification of tau filaments. Fibrous core regions of tau filaments extracted from brains of AD, PiD, PSP and CBD patients identified by cryogenic electron microscopic studies explain the templated tau amplification and tau filament formation observed in our cellular model. The core region of AD-tau (G273-E380 in 3R tau and G304-E380 in 4R tau), which recruits both 3R tau and 4R tau for seeded aggregation, consists of amino acid sequences common to 3R tau and 4R tau. On the other hand, PiD-tau, PSP-tau and CBD-tau consist of 3R tau or 4R tau specific amino acid sequences: K254-F378 in 3R tau, G272-N381 in 4R tau and K274-E380 in 4R tau. Therefore, PiD-tau, PSP-tau and CBD-tau recruit only tau substrates that match the template for seeded aggregation, and do not induce aggregation when the template and substrate are mismatched. The amplification and intracerebral expansion of tau filaments with the same structure by this mechanism leads to the pathological diversity of tauopathies
The requirement of amino acid sequence identity between template and substrate for template-dependent amplification is also clearly demonstrated by the in vitro and in vivo observation of low seeding and propagation efficiency caused by species barriers [191, 251]. Seven residues in 140 amino acids differ between the amino acid sequences of human and mouse α-syn, and it has been reported that the seeding activity is significantly decreased when the substrate and template are from different species [191]. This is probably due to structural instability resulting from the mismatch between template and substrate. Although the mechanism by which distinct strains are generated remains unclear, distinct folds (templates) are formed from normal proteins, depending on genetic and/or environmental factors (Fig. 5a). Then, in the presence of a substrate matching the template (and cofactor), filaments composed of the same fold are formed and amplified (Fig. 5a). Disease-associated missense mutations directly affect the fibrous core structure and generate different strains. Structural analysis of mutant synthetic α-syn filaments associated with familial synucleinopathies has demonstrated that these mutations affect not only the core structure, but also the interface between the two protofilaments [36, 37, 122, 186, 279, 280, 323]. As environmental factors, the brain region and cell types (neurons, astrocytes and oligodendrocytes) in which aggregation initially occurs may contribute substantially to strain formation. Patient-derived pathogenic proteins have been shown experimentally to retain their properties even during amplification in different cellular environments [228]. The formation of 3R or 4R tau-specific strains in tauopathies may be caused by the heterogeneous expression of tau isoforms depending on the brain region and the cell type [54, 96, 195]. Oligodendrocyte-specific tubulin polymerization-promoting protein, p25α, is mislocalized to the cell body prior to α-syn accumulation in MSA cases, and it has been suggested that p25α may contribute to the formation of MSA-specific strains [96]. Differences in the aging process in each cell type may also be a factor. In addition, cell type-specific or non-specific cofactors could be directly responsible for the formation of disease-specific folds. Potential cofactors within the CTE and CBD folds include lipids and polyanions, which have been experimentally found to affect filament formation [86, 91, 97, 116, 322]. The involvement of PTMs in strain formation is supported by the combination of structural and mass analysis, which suggests that the ubiquitination at K340 that is characteristic of singlet-type CBD filaments prevents doublet formation [18]. As experimentally demonstrated, other proteins, lipids and nucleic acids interacting with prion-like proteins, salt concentration, pH and metals are also environment factors causing various forms of misfolding [12, 35, 57, 58, 117, 142, 150, 158, 270]. Once the filaments accumulate in the cell, the fragmented pathogenic seeds are released into the extracellular space and taken up by neighboring healthy cells. Although the mechanism of cell-to-cell transmission is not fully understood, the pathogenic seeds may be released through exocytosis or transport mediated by membrane vesicles, including synaptic vesicles and exosomes (Fig. 5b) [8, 60, 189, 217, 234, 254]. The leakage of pathogenic seeds from dead cells is also feasible. Extracellular pathogenic seeds may then be taken up into the cell directly, or by macropinocytosis, receptor-mediated endocytosis, or membrane fusion of exosomes (Fig. 5b) [31, 93, 100, 303]. Recently, it has also been demonstrated that pathogenic seeds can pass between neuronal cells via tunneling nanotubes (Fig. 5b) [2, 82, 285]. The continuous cell-to-cell transmission of these pathogenic proteins along the neuronal circuits leads to the intracerebral expansion of the pathology. A disease-specific fold is maintained during the amplification and spreading of pathogenic protein in the brain, resulting in the clinical and pathological diversity. Although the structures of α-syn filaments amplified by in vitro assays using brain and CSF samples derived from patients with synucleinopathies have been revealed by cryo-EM analysis, these structures are not identical to those of α-syn filaments extracted from patients’ brains [51, 190]. Therefore, the cellular environment and cofactors may play critical roles in strain formation.
Strain formation and cell-to-cell transmission in neurodegenerative diseases. a Prion-like proteins, including tau, α-syn and TDP-43, can adopt various misfolded forms. The variety of misfolding is caused by genetic and/or environmental factors, resulting in the formation of strains with distinct conformations. Disease-associated mutations alter the core structure of filaments and the interaction between two protofilaments. Differences in the cellular environment between neuronal and glial cells may also contribute to the various types of misfolding. The interaction of prion-like proteins with cell type-specific co-factors during misfolding would lead to the formation of disease-specific filaments. Post-translational modifications, interactions with other proteins, lipids and nucleic acids, as well as differences in salt concentration, pH and metals, may also be involved in the formation of distinct strains. b Pathogenic proteins amplified and accumulated in cells have proposed to transmit as seeds from cell to cell and then spread throughout the brain. Possible mechanisms of cell-to-cell transmission include extracellular release of pathogenic seeds via exocytosis or in synaptic vesicles or exosomes, followed by incorporation into neighboring cells either directly or via macropinocytosis or receptor-mediated endocytosis. Alternatively pathogenic seeds may be taken up into cells by cell membrane fusion of exosomes containing seeds. Cell-to-cell transmission of pathogenic seeds via tunneling nanotubes has also been suggested
Directions for future research
The heterogeneity of abnormal proteins accumulated in patients’ brains is a key factor in the onset and progression of neurodegenerative diseases. Structural and biochemical analyses of post-mortem brains have revealed structural polymorphisms (strains) and prion-like properties of pathogenic proteins. Notably, recent cryo-EM studies of tau and α-syn filaments extracted from patients’ brains have provided direct evidence of strain formation in the brain (Table 1). Detailed structural information about these pathogenic proteins will contribute to the development of disease-modifying agents targeting protein aggregation and cell-to-cell transmission [261]. We believe there will be an increasing focus on the fibrous core structure in the design of aggregation inhibitors and antibodies for antibody therapy. The differences in the disease-specific fibrous core structures may also indicate that different approaches will be required for each disease. The same can be expected to apply to PET ligands used for early diagnosis, and this may lead to the development of PET ligands that can distinguish each disease within the same proteinopathy. Cryo-EM analysis has revealed the binding sites of EGCG, a natural compound that inhibits amyloid formation, and APN-1607, a PET ligand known as PM-PBB3, to tau filaments extracted from AD cases [260, 265]. Elucidation of the mechanisms underlying strain formation, as well as related factors, is crucial to reach a better understanding of the pathogenic protein strains in neurodegenerative diseases. The matured filaments that define each disease are supposed to pass through several different morphological forms during the maturation process. It will be important to clarify whether the disease-specific fold is established at the point of initial fibrillar species formation or at some other point during the maturation process, both in order to elucidate the mechanism of the strain formation and to characterize the pathological transition from pre-tangles/pre-inclusions to fibrillar inclusions. Recently, comprehensive proteomic analysis has identified a number of proteins that interact with tau, α-syn and TDP-43 [99, 123, 181, 292]. A similar approach would be useful to identify cell type-specific proteins that interact with pathogenic proteins and are involved in strain formation. In addition, the co-occurrence of multiple pathogenic proteins in the same patient is common in neurodegenerative diseases, but it is not fully understood how the interactions of these proteins contribute to the pathogenesis [146, 202]. Phosphorylated TDP-43 has been shown to co-localize in NFTs in AD and LBs in LBD, which may indicate a direct contribution of TDP-43 to AD and LBD pathogenesis [17, 138]. It has been also reported that distinct α-syn strains exhibit different abilities to induce tau aggregation and that the frequency of TDP-43 co-occurrence in tauopathies varies among diseases [124, 298]. The ways in which the interactions of multiple pathogenic proteins contribute to pathogenesis and the strain-specific characteristics of the interactions require further investigation. Structural analysis using cryo-EM will continue to be indispensable for the study of neurodegenerative diseases. It will be intriguing to see whether the abnormal proteins amplified in cellular and animal models inherit the structure of the patient-derived filaments used as the original seeds. In situ visualization of cultured cells and primary cultures with aggregates using cryo-EM tomography has also been reported, and analysis of brain samples from animals and patients using this approach may be fruitful [29, 126, 293]. Now that the importance of the cellular environment in strain formation has been suggested, understanding the changes in the cellular dynamics of cells with aggregates would be helpful to elucidate not only the mechanisms of aggregation, cell-to-cell transmission and neurodegeneration, but also the mechanism of strain formation.
Change history
20 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00401-022-02439-y
References
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252. https://doi.org/10.1016/s0896-6273(00)80886-7
Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L et al (2016) Tunneling nanotubes spread fibrillar alpha-synuclein by intercellular trafficking of lysosomes. EMBO J 35:2120–2138. https://doi.org/10.15252/embj.201593411
Aguilar-Calvo P, Xiao X, Bett C, Erana H, Soldau K, Castilla J et al (2017) Post-translational modifications in PrP expand the conformational diversity of prions in vivo. Sci Rep 7:43295. https://doi.org/10.1038/srep43295
Ahmed Z, Doherty KM, Silveira-Moriyama L, Bandopadhyay R, Lashley T, Mamais A et al (2011) Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol 122:415–428. https://doi.org/10.1007/s00401-011-0857-4
Al-Hilaly YK, Foster BE, Biasetti L, Lutter L, Pollack SJ, Rickard JE et al (2020) Tau (297–391) forms filaments that structurally mimic the core of paired helical filaments in Alzheimer’s disease brain. FEBS Lett 594:944–950. https://doi.org/10.1002/1873-3468.13675
Al-Hilaly YK, Pollack SJ, Vadukul DM, Citossi F, Rickard JE, Simpson M et al (2017) Alzheimer’s disease-like paired helical filament assembly from truncated tau protein is independent of disulfide crosslinking. J Mol Biol 429:3650–3665. https://doi.org/10.1016/j.jmb.2017.09.007
Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SSW et al (2014) Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81:536–543. https://doi.org/10.1016/j.neuron.2013.12.018
Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ et al (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42:360–367. https://doi.org/10.1016/j.nbd.2011.01.029
Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ et al (2006) Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281:29739–29752. https://doi.org/10.1074/jbc.M600933200
Anderson P, Kedersha N (2008) Stress granules: the Tao of RNA triage. Trends Biochem Sci 33:141–150. https://doi.org/10.1016/j.tibs.2007.12.003
Anderton BH, Breinburg D, Downes MJ, Green PJ, Tomlinson BE, Ulrich J et al (1982) Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature 298:84–86. https://doi.org/10.1038/298084a0
Antonschmidt L, Dervisoglu R, Sant V, Tekwani Movellan K, Mey I, Riedel D et al (2021) Insights into the molecular mechanism of amyloid filament formation: segmental folding of alpha-synuclein on lipid membranes. Sci Adv. https://doi.org/10.1126/sciadv.abg2174
Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B et al (2013) Alpha-synuclein p. H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord 28:811–813. https://doi.org/10.1002/mds.25421
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611. https://doi.org/10.1016/j.bbrc.2006.10.093
Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K et al (2004) Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79. https://doi.org/10.1002/ana.10793
Arai T, Ikeda K, Akiyama H, Tsuchiya K, Iritani S, Ishiguro K et al (2003) Different immunoreactivities of the microtubule-binding region of tau and its molecular basis in brains from patients with Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 105:489–498. https://doi.org/10.1007/s00401-003-0671-8
Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K et al (2009) Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 117:125–136. https://doi.org/10.1007/s00401-008-0480-1
Arakhamia T, Lee CE, Carlomagno Y, Duong DM, Kundinger SR, Wang K et al (2020) Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell 180(633–644):e612. https://doi.org/10.1016/j.cell.2020.01.027
Arima K (2006) Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 26:475–483. https://doi.org/10.1111/j.1440-1789.2006.00669.x
Arima K, Oyanagi S, Kosaka K, Matsushita M (1987) Distribution of Pick bodies in the central nervous system of Pick’s disease with special reference to their association with neuronal loss. Seishin Shinkeigaku Zasshi 89:43–72
Arima K, Uesugi H, Fujita I, Sakurai Y, Oyanagi S, Andoh S et al (1994) Corticonigral degeneration with neuronal achromasia presenting with primary progressive aphasia: ultrastructural and immunocytochemical studies. J Neurol Sci 127:186–197. https://doi.org/10.1016/0022-510x(94)90072-8
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631–639. https://doi.org/10.1212/wnl.42.3.631
Arseni D, Hasegawa M, Murzin AG, Kametani F, Arai M, Yoshida M et al (2021) Structure of pathological TDP-43 filaments from ALS with FTLD. Nature. https://doi.org/10.1038/s41586-021-04199-3
Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D et al (2011) Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 17:175–178. https://doi.org/10.1038/nm.2294
Ayala YM, Zago P, D’Ambrogio A, Xu YF, Petrucelli L, Buratti E et al (2008) Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci 121:3778–3785. https://doi.org/10.1242/jcs.038950
Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM et al (1998) Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152:879–884
Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G et al (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res 477:90–99. https://doi.org/10.1016/0006-8993(89)91396-6
Barrett PJ, Timothy Greenamyre J (2015) Post-translational modification of alpha-synuclein in Parkinson’s disease. Brain Res 1628:247–253. https://doi.org/10.1016/j.brainres.2015.06.002
Bauerlein FJB, Saha I, Mishra A, Kalemanov M, Martinez-Sanchez A, Klein R et al (2017) In situ architecture and cellular interactions of PolyQ inclusions. Cell 171(179–187):e110. https://doi.org/10.1016/j.cell.2017.08.009
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL et al (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702. https://doi.org/10.1007/s00401-010-0664-3
Ben Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe DJ, Sharon R (2009) Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic 10:218–234. https://doi.org/10.1111/j.1600-0854.2008.00853.x
Bendheim PE, Bockman JM, McKinley MP, Kingsbury DT, Prusiner SB (1985) Scrapie and Creutzfeldt–Jakob disease prion proteins share physical properties and antigenic determinants. Proc Natl Acad Sci USA 82:997–1001. https://doi.org/10.1073/pnas.82.4.997
Black MM, Slaughter T, Moshiach S, Obrocka M, Fischer I (1996) Tau is enriched on dynamic microtubules in the distal region of growing axons. J Neurosci 16:3601–3619
Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D et al (2000) Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson’s disease and normal subjects. Neurosci Lett 287:65–67. https://doi.org/10.1016/s0304-3940(00)01153-8
Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B et al (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575. https://doi.org/10.1038/ncomms3575
Boyer DR, Li B, Sun C, Fan W, Sawaya MR, Jiang L et al (2019) Structures of fibrils formed by alpha-synuclein hereditary disease mutant H50Q reveal new polymorphs. Nat Struct Mol Biol 26:1044–1052. https://doi.org/10.1038/s41594-019-0322-y
Boyer DR, Li B, Sun C, Fan W, Zhou K, Hughes MP et al (2020) The alpha-synuclein hereditary mutation E46K unlocks a more stable, pathogenic fibril structure. Proc Natl Acad Sci USA 117:3592–3602. https://doi.org/10.1073/pnas.1917914117
Braak H, Braak E (1987) Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett 76:124–127. https://doi.org/10.1016/0304-3940(87)90204-7
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/BF00308809
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Braak H, Rub U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 110:517–536. https://doi.org/10.1007/s00702-002-0808-2
Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. https://doi.org/10.1097/NEN.0b013e318232a379
Brandmeir NJ, Geser F, Kwong LK, Zimmerman E, Qian J, Lee VM et al (2008) Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol 115:123–131. https://doi.org/10.1007/s00401-007-0315-5
Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB et al (2014) Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol 127:423–439. https://doi.org/10.1007/s00401-013-1238-y
Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M et al (2013) Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74:20–38. https://doi.org/10.1002/ana.23937
Brion J, Passareiro H, Nunez J, Flament-Durand J (1985) Mise en evidence immunologique de la proteine tau au niveau des lesions de degenerescence neurofibrillaire de la maladie d’Alzheimer. Arch Biol (Bruxelles) 95:229–235
Brion JP, Guilleminot J, Couchie D, Flament-Durand J, Nunez J (1988) Both adult and juvenile tau microtubule-associated proteins are axon specific in the developing and adult rat cerebellum. Neuroscience 25:139–146. https://doi.org/10.1016/0306-4522(88)90013-9
Buratti E, Baralle FE (2001) Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem 276:36337–36343. https://doi.org/10.1074/jbc.M104236200
Buratti E, Baralle FE (2008) Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci 13:867–878. https://doi.org/10.2741/2727
Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE (2001) Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J 20:1774–1784. https://doi.org/10.1093/emboj/20.7.1774
Burger D, Fenyi A, Bousset L, Stahlberg H, Melki R (2021) Cryo-EM structure of alpha-synuclein fibrils amplified by PMCA from PD and MSA patient brains. bioRxiv. https://doi.org/10.1101/2021.07.08.451588
Burke CM, Walsh DJ, Mark KMK, Deleault NR, Nishina KA, Agrimi U et al (2020) Cofactor and glycosylation preferences for in vitro prion conversion are predominantly determined by strain conformation. PLoS Pathog 16:e1008495. https://doi.org/10.1371/journal.ppat.1008495
Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329:1663–1667. https://doi.org/10.1126/science.1195227
Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R (2006) Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet 15:3529–3537. https://doi.org/10.1093/hmg/ddl429
Campbell BC, McLean CA, Culvenor JG, Gai WP, Blumbergs PC, Jakala P et al (2001) The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem 76:87–96. https://doi.org/10.1046/j.1471-4159.2001.00021.x
Cao Q, Boyer DR, Sawaya MR, Ge P, Eisenberg DS (2019) Cryo-EM structures of four polymorphic TDP-43 amyloid cores. Nat Struct Mol Biol 26:619–627. https://doi.org/10.1038/s41594-019-0248-4
Caragounis A, Price KA, Soon CP, Filiz G, Masters CL, Li QX et al (2010) Zinc induces depletion and aggregation of endogenous TDP-43. Free Radic Biol Med 48:1152–1161. https://doi.org/10.1016/j.freeradbiomed.2010.01.035
Castellani RJ, Siedlak SL, Perry G, Smith MA (2000) Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol 100:111–114. https://doi.org/10.1007/s004010050001
Castilla J, Saa P, Morales R, Abid K, Maundrell K, Soto C (2006) Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol 412:3–21. https://doi.org/10.1016/S0076-6879(06)12001-7
Chai X, Dage JL, Citron M (2012) Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol Dis 48:356–366. https://doi.org/10.1016/j.nbd.2012.05.021
Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X et al (2004) Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA 101:14966–14971. https://doi.org/10.1073/pnas.0406283101
Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123:383–396. https://doi.org/10.1016/j.cell.2005.09.028
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S et al (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169. https://doi.org/10.1016/S0140-6736(04)17103-1
Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA et al (2004) Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett 576:363–368. https://doi.org/10.1016/j.febslet.2004.09.038
Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B et al (1998) Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci USA 95:13103–13107. https://doi.org/10.1073/pnas.95.22.13103
Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J et al (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540. https://doi.org/10.1073/pnas.1301175110
Cleveland DW, Hwo SY, Kirschner MW (1977) Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol 116:227–247. https://doi.org/10.1016/0022-2836(77)90214-5
Cleveland DW, Hwo SY, Kirschner MW (1977) Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol 116:207–225. https://doi.org/10.1016/0022-2836(77)90213-3
Colombrita C, Onesto E, Megiorni F, Pizzuti A, Baralle FE, Buratti E et al (2012) TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J Biol Chem 287:15635–15647. https://doi.org/10.1074/jbc.M111.333450
Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E et al (2009) TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem 111:1051–1061. https://doi.org/10.1111/j.1471-4159.2009.06383.x
Corbett GT, Wang Z, Hong W, Colom-Cadena M, Rose J, Liao M et al (2020) PrP is a central player in toxicity mediated by soluble aggregates of neurodegeneration-causing proteins. Acta Neuropathol 139:503–526. https://doi.org/10.1007/s00401-019-02114-9
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. https://doi.org/10.1007/s00401-014-1349-0
Crowther RA (1991) Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proc Natl Acad Sci USA 88:2288–2292. https://doi.org/10.1073/pnas.88.6.2288
D’Souza I, Poorkaj P, Hong M, Nochlin D, Lee VM, Bird TD et al (1999) Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci USA 96:5598–5603. https://doi.org/10.1073/pnas.96.10.5598
Dage JL, Wennberg AMV, Airey DC, Hagen CE, Knopman DS, Machulda MM et al (2016) Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement 12:1226–1234. https://doi.org/10.1016/j.jalz.2016.06.001
Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN (1992) Relationships between Lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol 83:525–529. https://doi.org/10.1007/BF00310030
Dan A, Takahashi M, Masuda-Suzukake M, Kametani F, Nonaka T, Kondo H et al (2013) Extensive deamidation at asparagine residue 279 accounts for weak immunoreactivity of tau with RD4 antibody in Alzheimer’s disease brain. Acta Neuropathol Commun 1:54. https://doi.org/10.1186/2051-5960-1-54
Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D et al (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533. https://doi.org/10.1007/s00401-006-0189-y
De Rossi P, Lewis AJ, Furrer J, De Vos L, Demeter T, Zbinden A et al (2021) FTLD-TDP assemblies seed neoaggregates with subtype-specific features via a prion-like cascade. EMBO Rep 22:e53877. https://doi.org/10.15252/embr.202153877
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. https://doi.org/10.1016/j.neuron.2011.09.011
Dewey CM, Cenik B, Sephton CF, Johnson BA, Herz J, Yu G (2012) TDP-43 aggregation in neurodegeneration: are stress granules the key? Brain Res 1462:16–25. https://doi.org/10.1016/j.brainres.2012.02.032
Ding X, Ma M, Teng J, Teng RK, Zhou S, Yin J et al (2015) Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure. Oncotarget 6:24178–24191. https://doi.org/10.18632/oncotarget.4680
Duffy PE, Tennyson VM (1965) Phase and electron microscopic observations of lewy bodies and melanin granules in the substantia nigra and locus caeruleus in Parkinson’s disease. J Neuropathol Exp Neurol 24:398–414
El-Agnaf OM, Jakes R, Curran MD, Wallace A (1998) Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of alpha-synuclein protein implicated in Parkinson’s disease. FEBS Lett 440:67–70. https://doi.org/10.1016/s0014-5793(98)01419-7
El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ et al (2003) Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J 17:1945–1947. https://doi.org/10.1096/fj.03-0098fje
Elbaum-Garfinkle S, Ramlall T, Rhoades E (2010) The role of the lipid bilayer in tau aggregation. Biophys J 98:2722–2730. https://doi.org/10.1016/j.bpj.2010.03.013
Emmenegger M, De Cecco E, Hruska-Plochan M, Eninger T, Schneider MM, Barth M et al (2021) LAG3 is not expressed in human and murine neurons and does not modulate alpha-synucleinopathies. EMBO Mol Med 13:e14745. https://doi.org/10.15252/emmm.202114745
Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S et al (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3:812–818. https://doi.org/10.1002/acn3.338
Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R et al (2018) Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561:137–140. https://doi.org/10.1038/s41586-018-0454-y
Falcon B, Zhang W, Schweighauser M, Murzin AG, Vidal R, Garringer HJ et al (2018) Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathol 136:699–708. https://doi.org/10.1007/s00401-018-1914-z
Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R et al (2019) Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568:420–423. https://doi.org/10.1038/s41586-019-1026-5
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM et al (2015) TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol 211:897–911. https://doi.org/10.1083/jcb.201504057
Ferese R, Modugno N, Campopiano R, Santilli M, Zampatti S, Giardina E et al (2015) Four copies of SNCA responsible for autosomal dominant Parkinson’s disease in two Italian siblings. Parkinsons Dis 2015:546462. https://doi.org/10.1155/2015/546462
Fernandes N, Eshleman N, Buchan JR (2018) Stress granules and ALS: a case of causation or correlation? Adv Neurobiol 20:173–212. https://doi.org/10.1007/978-3-319-89689-2_7
Ferreira N, Gram H, Sorrentino ZA, Gregersen E, Schmidt SI, Reimer L et al (2021) Multiple system atrophy-associated oligodendroglial protein p25alpha stimulates formation of novel alpha-synuclein strain with enhanced neurodegenerative potential. Acta Neuropathol 142:87–115. https://doi.org/10.1007/s00401-021-02316-0
Fichou Y, Lin Y, Rauch JN, Vigers M, Zeng Z, Srivastava M et al (2018) Cofactors are essential constituents of stable and seeding-active tau fibrils. Proc Natl Acad Sci USA 115:13234–13239. https://doi.org/10.1073/pnas.1810058115
Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ et al (2017) Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547:185–190. https://doi.org/10.1038/nature23002
Freibaum BD, Chitta RK, High AA, Taylor JP (2010) Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. J Proteome Res 9:1104–1120. https://doi.org/10.1021/pr901076y
Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284:12845–12852. https://doi.org/10.1074/jbc.M808759200
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS et al (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. https://doi.org/10.1038/ncb748
Gambetti P, Dong Z, Yuan J, Xiao X, Zheng M, Alshekhlee A et al (2008) A novel human disease with abnormal prion protein sensitive to protease. Ann Neurol 63:697–708. https://doi.org/10.1002/ana.21420
Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M (2015) Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol Appl Neurobiol 41:24–46. https://doi.org/10.1111/nan.12213
Giasson BI, Uryu K, Trojanowski JQ, Lee VM (1999) Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 274:7619–7622. https://doi.org/10.1074/jbc.274.12.7619
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676. https://doi.org/10.1212/01.wnl.0000324625.00404.15
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890. https://doi.org/10.1016/s0006-291x(84)80190-4
Goedert M, Crowther RA, Garner CC (1991) Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci 14:193–199. https://doi.org/10.1016/0166-2236(91)90105-4
Goedert M, Crowther RA, Spillantini MG (1998) Tau mutations cause frontotemporal dementias. Neuron 21:955–958. https://doi.org/10.1016/s0896-6273(00)80615-7
Goedert M, Jakes R (1990) Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J 9:4225–4230
Goedert M, Jakes R, Crowther RA (1999) Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett 450:306–311. https://doi.org/10.1016/s0014-5793(99)00508-6
Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA (1996) Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 383:550–553. https://doi.org/10.1038/383550a0
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168. https://doi.org/10.1016/0896-6273(92)90117-v
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3:519–526. https://doi.org/10.1016/0896-6273(89)90210-9
Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC et al (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 41:17–24. https://doi.org/10.1002/ana.410410106
Gomez-Tortosa E, Newell K, Irizarry MC, Sanders JL, Hyman BT (2000) alpha-Synuclein immunoreactivity in dementia with Lewy bodies: morphological staging and comparison with ubiquitin immunostaining. Acta Neuropathol 99:352–357. https://doi.org/10.1007/s004010051135
Goux WJ, Liu B, Shumburo AM, Parikh S, Sparkman DR (2001) A quantitative assessment of glycolipid and protein associated with paired helical filament preparations from Alzheimer’s diseased brain. J Alzheimers Dis 3:455–466. https://doi.org/10.3233/jad-2001-3504
Goux WJ, Rodriguez S, Sparkman DR (1995) Analysis of the core components of Alzheimer paired helical filaments. A gas chromatography/mass spectrometry characterization of fatty acids, carbohydrates and long-chain bases. FEBS Lett 366:81–85. https://doi.org/10.1016/0014-5793(95)00486-s
Gribaudo S, Tixador P, Bousset L, Fenyi A, Lino P, Melki R et al (2019) Propagation of alpha-synuclein strains within human reconstructed neuronal network. Stem Cell Rep 12:230–244. https://doi.org/10.1016/j.stemcr.2018.12.007
Grinberg LT, Wang X, Wang C, Sohn PD, Theofilas P, Sidhu M et al (2013) Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol 125:581–593. https://doi.org/10.1007/s00401-013-1080-2
Grover A, Houlden H, Baker M, Adamson J, Lewis J, Prihar G et al (1999) 5’ splice site mutations in tau associated with the inherited dementia FTDP-17 affect a stem-loop structure that regulates alternative splicing of exon 10. J Biol Chem 274:15134–15143. https://doi.org/10.1074/jbc.274.21.15134
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917. https://doi.org/10.1073/pnas.83.13.4913
Guerrero-Ferreira R, Taylor NM, Arteni AA, Kumari P, Mona D, Ringler P et al (2019) Two new polymorphic structures of human full-length alpha-synuclein fibrils solved by cryo-electron microscopy. Elife. https://doi.org/10.7554/eLife.48907
Gunawardana CG, Mehrabian M, Wang X, Mueller I, Lubambo IB, Jonkman JE et al (2015) The human tau interactome: binding to the ribonucleoproteome, and impaired binding of the proline-to-leucine mutant at position 301 (P301L) to chaperones and the proteasome. Mol Cell Proteom 14:3000–3014. https://doi.org/10.1074/mcp.M115.050724
Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B et al (2013) Distinct alpha-synuclein strains differentially promote tau inclusions in neurons. Cell 154:103–117. https://doi.org/10.1016/j.cell.2013.05.057
Guo JL, Narasimhan S, Changolkar L, He Z, Stieber A, Zhang B et al (2016) Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J Exp Med 213:2635–2654. https://doi.org/10.1084/jem.20160833
Guo Q, Lehmer C, Martinez-Sanchez A, Rudack T, Beck F, Hartmann H et al (2018) In situ structure of neuronal C9orf72 Poly-GA aggregates reveals proteasome recruitment. Cell 172(696–705):e612. https://doi.org/10.1016/j.cell.2017.12.030
Hallinan GI, Hoq MR, Ghosh M, Vago FS, Fernandez A, Garringer HJ et al (2021) Structure of Tau filaments in Prion protein amyloidoses. Acta Neuropathol 142:227–241. https://doi.org/10.1007/s00401-021-02336-w
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y et al (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70. https://doi.org/10.1002/ana.21425
Hasegawa M, Crowther RA, Jakes R, Goedert M (1997) Alzheimer-like changes in microtubule-associated protein Tau induced by sulfated glycosaminoglycans. Inhibition of microtubule binding, stimulation of phosphorylation, and filament assembly depend on the degree of sulfation. J Biol Chem 272:33118–33124. https://doi.org/10.1074/jbc.272.52.33118
Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM et al (2002) Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem 277:49071–49076. https://doi.org/10.1074/jbc.M208046200
Hasegawa M, Nonaka T, Tsuji H, Tamaoka A, Yamashita M, Kametani F et al (2011) Molecular dissection of TDP-43 proteinopathies. J Mol Neurosci 45:480–485. https://doi.org/10.1007/s12031-011-9571-x
Hasegawa M, Smith MJ, Goedert M (1998) Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 437:207–210. https://doi.org/10.1016/s0014-5793(98)01217-4
Hashimoto M, Yoshimoto M, Sisk A, Hsu LJ, Sundsmo M, Kittel A et al (1997) NACP, a synaptic protein involved in Alzheimer’s disease, is differentially regulated during megakaryocyte differentiation. Biochem Biophys Res Commun 237:611–616. https://doi.org/10.1006/bbrc.1997.6978
Hauw JJ, Verny M, Delaere P, Cervera P, He Y, Duyckaerts C (1990) Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer’s disease and aging. Neurosci Lett 119:182–186. https://doi.org/10.1016/0304-3940(90)90829-x
Hawkes CH, Del Tredici K, Braak H (2010) A timeline for Parkinson’s disease. Parkinsonism Relat Disord 16:79–84. https://doi.org/10.1016/j.parkreldis.2009.08.007
He Z, McBride JD, Xu H, Changolkar L, Kim SJ, Zhang B et al (2020) Transmission of tauopathy strains is independent of their isoform composition. Nat Commun 11:7. https://doi.org/10.1038/s41467-019-13787-x
Head MW, Yull HM, Ritchie DL, Langeveld JP, Fletcher NA, Knight RS et al (2013) Variably protease-sensitive prionopathy in the UK: a retrospective review 1991–2008. Brain 136:1102–1115. https://doi.org/10.1093/brain/aws366
Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K et al (2007) Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294. https://doi.org/10.1016/j.brainres.2007.09.048
Hill AF, Joiner S, Wadsworth JD, Sidle KC, Bell JE, Budka H et al (2003) Molecular classification of sporadic Creutzfeldt–Jakob disease. Brain 126:1333–1346. https://doi.org/10.1093/brain/awg125
Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K et al (2013) Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci USA 110:E3138-3147. https://doi.org/10.1073/pnas.1301440110
Horio T, Hotani H (1986) Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature 321:605–607. https://doi.org/10.1038/321605a0
Hoyer W, Antony T, Cherny D, Heim G, Jovin TM, Subramaniam V (2002) Dependence of alpha-synuclein aggregate morphology on solution conditions. J Mol Biol 322:383–393. https://doi.org/10.1016/s0022-2836(02)00775-1
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H et al (1998) Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705. https://doi.org/10.1038/31508
Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P et al (2004) Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet 364:1169–1171. https://doi.org/10.1016/S0140-6736(04)17104-3
Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE et al (2016) Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol 79:272–287. https://doi.org/10.1002/ana.24559
Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX et al (2017) Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 16:55–65. https://doi.org/10.1016/S1474-4422(16)30291-5
Iseki E, Li F, Odawara T, Hino H, Suzuki K, Kosaka K et al (1998) Ubiquitin-immunohistochemical investigation of atypical Pick’s disease without Pick bodies. J Neurol Sci 159:194–201. https://doi.org/10.1016/s0022-510x(98)00168-3
Jakes R, Spillantini MG, Goedert M (1994) Identification of two distinct synucleins from human brain. FEBS Lett 345:27–32. https://doi.org/10.1016/0014-5793(94)00395-5
Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE et al (2020) Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 26:379–386. https://doi.org/10.1038/s41591-020-0755-1
Jeganathan S, von Bergen M, Mandelkow EM, Mandelkow E (2008) The natively unfolded character of tau and its aggregation to Alzheimer-like paired helical filaments. Biochemistry 47:10526–10539. https://doi.org/10.1021/bi800783d
Jensen M, Basun H, Lannfelt L (1995) Increased cerebrospinal fluid tau in patients with Alzheimer’s disease. Neurosci Lett 186:189–191. https://doi.org/10.1016/0304-3940(95)11297-a
Jin H, Ishikawa K, Tsunemi T, Ishiguro T, Amino T, Mizusawa H (2008) Analyses of copy number and mRNA expression level of the alpha-synuclein gene in multiple system atrophy. J Med Dent Sci 55:145–153
Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE (2000) alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem 275:34328–34334. https://doi.org/10.1074/jbc.M004345200
Johnson JO, Pioro EP, Boehringer A, Chia R, Feit H, Renton AE et al (2014) Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat Neurosci 17:664–666. https://doi.org/10.1038/nn.3688
Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574. https://doi.org/10.1038/ng.132
Kametani F, Obi T, Shishido T, Akatsu H, Murayama S, Saito Y et al (2016) Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains. Sci Rep 6:23281. https://doi.org/10.1038/srep23281
Kametani F, Yoshida M, Matsubara T, Murayama S, Saito Y, Kawakami I et al (2020) Comparison of common and disease-specific post-translational modifications of pathological tau associated with a wide range of tauopathies. Front Neurosci 14:581936. https://doi.org/10.3389/fnins.2020.581936
Kampers T, Friedhoff P, Biernat J, Mandelkow EM, Mandelkow E (1996) RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett 399:344–349. https://doi.org/10.1016/s0014-5793(96)01386-5
Kapasi A, Brosch JR, Nudelman KN, Agrawal S, Foroud TM, Schneider JA (2020) A novel SNCA E83Q mutation in a case of dementia with Lewy bodies and atypical frontotemporal lobar degeneration. Neuropathology 40:620–626. https://doi.org/10.1111/neup.12687
Kapeli K, Martinez FJ, Yeo GW (2017) Genetic mutations in RNA-binding proteins and their roles in ALS. Hum Genet 136:1193–1214. https://doi.org/10.1007/s00439-017-1830-7
Katsinelos T, Zeitler M, Dimou E, Karakatsani A, Muller HM, Nachman E et al (2018) Unconventional secretion mediates the trans-cellular spreading of tau. Cell Rep 23:2039–2055. https://doi.org/10.1016/j.celrep.2018.04.056
Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM et al (2016) Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 92:796–812. https://doi.org/10.1016/j.neuron.2016.09.055
Kidd M (1963) Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 197:192–193. https://doi.org/10.1038/197192b0
Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z et al (2013) Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495:467–473. https://doi.org/10.1038/nature11922
Kinoshita A, Tomimoto H, Suenaga T, Akiguchi I, Kimura J (1997) Ubiquitin-related cytoskeletal abnormality in frontotemporal dementia: immunohistochemical and immunoelectron microscope studies. Acta Neuropathol 94:67–72. https://doi.org/10.1007/s004010050673
Kocisko DA, Priola SA, Raymond GJ, Chesebro B, Lansbury PT Jr, Caughey B (1995) Species specificity in the cell-free conversion of prion protein to protease-resistant forms: a model for the scrapie species barrier. Proc Natl Acad Sci USA 92:3923–3927. https://doi.org/10.1073/pnas.92.9.3923
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14:504–506. https://doi.org/10.1038/nm1747
Kosik KS, Joachim CL, Selkoe DJ (1986) Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA 83:4044–4048. https://doi.org/10.1073/pnas.83.11.4044
Kovacs GG, Majtenyi K, Spina S, Murrell JR, Gelpi E, Hoftberger R et al (2008) White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:963–975. https://doi.org/10.1097/NEN.0b013e318187a80f
Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C et al (2003) Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol 106:83–88. https://doi.org/10.1007/s00401-003-0705-2
Kraus A, Hoyt F, Schwartz CL, Hansen B, Artikis E, Hughson AG et al (2021) High-resolution structure and strain comparison of infectious mammalian prions. Mol Cell 81(4540–4551):e4546. https://doi.org/10.1016/j.molcel.2021.08.011
Kraus A, Saijo E, Metrick MA 2nd, Newell K, Sigurdson CJ, Zanusso G et al (2019) Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease. Acta Neuropathol 137:585–598. https://doi.org/10.1007/s00401-018-1947-3
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108. https://doi.org/10.1038/ng0298-106
Kuzkina A, Bargar C, Schmitt D, Rossle J, Wang W, Schubert AL et al (2021) Diagnostic value of skin RT-QuIC in Parkinson’s disease: a two-laboratory study. NPJ Parkinsons Dis 7:99. https://doi.org/10.1038/s41531-021-00242-2
La Vitola P, Beeg M, Balducci C, Santamaria G, Restelli E, Colombo L et al (2019) Cellular prion protein neither binds to alpha-synuclein oligomers nor mediates their detrimental effects. Brain 142:249–254. https://doi.org/10.1093/brain/awy318
Laferriere F, Maniecka Z, Perez-Berlanga M, Hruska-Plochan M, Gilhespy L, Hock EM et al (2019) TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat Neurosci 22:65–77. https://doi.org/10.1038/s41593-018-0294-y
Lau A, So RWL, Lau HHC, Sang JC, Ruiz-Riquelme A, Fleck SC et al (2020) alpha-Synuclein strains target distinct brain regions and cell types. Nat Neurosci 23:21–31. https://doi.org/10.1038/s41593-019-0541-x
Lavenir I, Passarella D, Masuda-Suzukake M, Curry A, Holton JL, Ghetti B et al (2019) Silver staining (Campbell-Switzer) of neuronal alpha-synuclein assemblies induced by multiple system atrophy and Parkinson’s disease brain extracts in transgenic mice. Acta Neuropathol Commun 7:148. https://doi.org/10.1186/s40478-019-0804-5
Lee EB, Porta S, Michael Baer G, Xu Y, Suh E, Kwong LK et al (2017) Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol 134:65–78. https://doi.org/10.1007/s00401-017-1679-9
Lee VM, Balin BJ, Otvos L Jr, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 251:675–678. https://doi.org/10.1126/science.1899488
Leitao ADG, Rudolffi-Soto P, Chappard A, Bhumkar A, Lau D, Hunter DJB et al (2021) Selectivity of Lewy body protein interactions along the aggregation pathway of alpha-synuclein. Commun Biol 4:1124. https://doi.org/10.1038/s42003-021-02624-x
Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N et al (2013) G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol 73:459–471. https://doi.org/10.1002/ana.23894
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ et al (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14:501–503. https://doi.org/10.1038/nm1746
Li Q, Babinchak WM, Surewicz WK (2021) Cryo-EM structure of amyloid fibrils formed by the entire low complexity domain of TDP-43. Nat Commun 12:1620. https://doi.org/10.1038/s41467-021-21912-y
Li W, Englund E, Widner H, Mattsson B, van Westen D, Latt J et al (2016) Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci USA 113:6544–6549. https://doi.org/10.1073/pnas.1605245113
Li Y, Zhao C, Luo F, Liu Z, Gui X, Luo Z et al (2018) Amyloid fibril structure of alpha-synuclein determined by cryo-electron microscopy. Cell Res 28:897–903. https://doi.org/10.1038/s41422-018-0075-x
Li YR, King OD, Shorter J, Gitler AD (2013) Stress granules as crucibles of ALS pathogenesis. J Cell Biol 201:361–372. https://doi.org/10.1083/jcb.201302044
Lin WL, Dickson DW (2008) Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol 116:205–213. https://doi.org/10.1007/s00401-008-0408-9
Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C et al (2012) Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7:e31302. https://doi.org/10.1371/journal.pone.0031302
Lovestam S, Schweighauser M, Matsubara T, Murayama S, Tomita T, Ando T et al (2021) Seeded assembly in vitro does not replicate the structures of alpha-synuclein filaments from multiple system atrophy. FEBS Open Bio 11:999–1013. https://doi.org/10.1002/2211-5463.13110
Luk KC, Covell DJ, Kehm VM, Zhang B, Song IY, Byrne MD et al (2016) Molecular and biological compatibility with host alpha-synuclein influences fibril pathogenicity. Cell Rep 16:3373–3387. https://doi.org/10.1016/j.celrep.2016.08.053
Mackenzie IR, Feldman H (2003) The relationship between extramotor ubiquitin-immunoreactive neuronal inclusions and dementia in motor neuron disease. Acta Neuropathol 105:98–102. https://doi.org/10.1007/s00401-002-0620-y
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. https://doi.org/10.1007/s00401-011-0845-8
Maiti NC, Apetri MM, Zagorski MG, Carey PR, Anderson VE (2004) Raman spectroscopic characterization of secondary structure in natively unfolded proteins: alpha-synuclein. J Am Chem Soc 126:2399–2408. https://doi.org/10.1021/ja0356176
Majounie E, Cross W, Newsway V, Dillman A, Vandrovcova J, Morris CM et al (2013) Variation in tau isoform expression in different brain regions and disease states. Neurobiol Aging 34:1922 e1907-1922 e1912. https://doi.org/10.1016/j.neurobiolaging.2013.01.017
Manka SW, Zhang W, Wenborn A, Betts J, Joiner S, Saibil H, Collinge J, Wadsworth JD (2021) 2.7 Å cryo-EM structure of ex vivo RML prion fibrils. bioRxiv https://doi.org/10.1101/2021.12.13.472424
Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y et al (2016) Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. https://doi.org/10.1126/science.aah3374
Maroteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8:2804–2815
Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H et al (2013) Prion-like spreading of pathological alpha-synuclein in brain. Brain 136:1128–1138. https://doi.org/10.1093/brain/awt037
Mattila PM, Roytta M, Torikka H, Dickson DW, Rinne JO (1998) Cortical Lewy bodies and Alzheimer-type changes in patients with Parkinson’s disease. Acta Neuropathol 95:576–582. https://doi.org/10.1007/s004010050843
Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E et al (2016) Plasma tau in Alzheimer disease. Neurology 87:1827–1835. https://doi.org/10.1212/WNL.0000000000003246
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2017) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol 27:472–479. https://doi.org/10.1111/bpa.12424
McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64. https://doi.org/10.1093/brain/aws307
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D et al (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89:88–100. https://doi.org/10.1212/WNL.0000000000004058
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. https://doi.org/10.1212/01.wnl.0000187889.17253.b1
Meyer RK, McKinley MP, Bowman KA, Braunfeld MB, Barry RA, Prusiner SB (1986) Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci USA 83:2310–2314. https://doi.org/10.1073/pnas.83.8.2310
Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB (2005) Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm (Vienna) 112:1613–1624. https://doi.org/10.1007/s00702-005-0378-1
Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR (2010) Clinical staging and disease progression in frontotemporal dementia. Neurology 74:1591–1597. https://doi.org/10.1212/WNL.0b013e3181e04070
Mohite GM, Kumar R, Panigrahi R, Navalkar A, Singh N, Datta D et al (2018) Comparison of kinetics, toxicity, oligomer formation, and membrane binding capacity of alpha-synuclein familial mutations at the a53 site, including the newly discovered A53V mutation. Biochemistry 57:5183–5187. https://doi.org/10.1021/acs.biochem.8b00314
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z et al (2015) Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85:296–302. https://doi.org/10.1016/j.neuron.2014.12.032
Mori F, Tanji K, Zhang HX, Nishihira Y, Tan CF, Takahashi H et al (2008) Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 116:193–203. https://doi.org/10.1007/s00401-008-0396-9
Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Watanabe A et al (1995) Hyperphosphorylation of tau in PHF. Neurobiol Aging 16:365–371. https://doi.org/10.1016/0197-4580(95)00027-c (discussion 371–380)
Narasimhan S, Guo JL, Changolkar L, Stieber A, McBride JD, Silva LV et al (2017) Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J Neurosci 37:11406–11423. https://doi.org/10.1523/JNEUROSCI.1230-17.2017
Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K et al (2019) Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142:1503–1527. https://doi.org/10.1093/brain/awz099
Neumann M, Frick P, Paron F, Kosten J, Buratti E, Mackenzie IR (2020) Antibody against TDP-43 phosphorylated at serine 375 suggests conformational differences of TDP-43 aggregates among FTLD-TDP subtypes. Acta Neuropathol 140:645–658. https://doi.org/10.1007/s00401-020-02207-w
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. https://doi.org/10.1126/science.1134108
Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T et al (2013) Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep 4:124–134. https://doi.org/10.1016/j.celrep.2013.06.007
Oesch B, Westaway D, Walchli M, McKinley MP, Kent SB, Aebersold R et al (1985) A cellular gene encodes scrapie PrP 27–30 protein. Cell 40:735–746. https://doi.org/10.1016/0092-8674(85)90333-2
Okamura Y, Kawakami I, Watanabe K, Oshima K, Niizato K, Ikeda K et al (2019) Tau progression in single severe frontal traumatic brain injury in human brains. J Neurol Sci 407:116495. https://doi.org/10.1016/j.jns.2019.116495
Olsson B, Portelius E, Cullen NC, Sandelius A, Zetterberg H, Andreasson U et al (2019) Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol 76:318–325. https://doi.org/10.1001/jamaneurol.2018.3746
Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB (1995) Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol 69:3584–3596. https://doi.org/10.1128/JVI.69.6.3584-3596.1995
Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D et al (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A 90:10962–10966. https://doi.org/10.1073/pnas.90.23.10962
Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100. https://doi.org/10.1016/0022-510x(89)90219-0
Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG et al (1996) Molecular basis of phenotypic variability in sporadic Creutzfeldt–Jakob disease. Ann Neurol 39:767–778. https://doi.org/10.1002/ana.410390613
Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J et al (2014) Novel alpha-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson’s disease-type pathology. Neurobiol Aging 35(2180):e2181-2185. https://doi.org/10.1016/j.neurobiolaging.2014.03.024
Peden AH, Sarode DP, Mulholland CR, Barria MA, Ritchie DL, Ironside JW et al (2014) The prion protein protease sensitivity, stability and seeding activity in variably protease sensitive prionopathy brain tissue suggests molecular overlaps with sporadic Creutzfeldt–Jakob disease. Acta Neuropathol Commun 2:152. https://doi.org/10.1186/s40478-014-0152-4
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M et al (2015) alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–344. https://doi.org/10.1038/nature14547
Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL et al (2018) Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature 557:558–563. https://doi.org/10.1038/s41586-018-0104-4
Perry G, Siedlak SL, Richey P, Kawai M, Cras P, Kalaria RN et al (1991) Association of heparan sulfate proteoglycan with the neurofibrillary tangles of Alzheimer’s disease. J Neurosci 11:3679–3683
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC et al (2004) UCSF chimera–—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Piccardo P, Dlouhy SR, Lievens PM, Young K, Bird TD, Nochlin D et al (1998) Phenotypic variability of Gerstmann–Straussler–Scheinker disease is associated with prion protein heterogeneity. J Neuropathol Exp Neurol 57:979–988. https://doi.org/10.1097/00005072-199810000-00010
Pollock NJ, Mirra SS, Binder LI, Hansen LA, Wood JG (1986) Filamentous aggregates in Pick’s disease, progressive supranuclear palsy, and Alzheimer’s disease share antigenic determinants with microtubule-associated protein, tau. Lancet 2:1211. https://doi.org/10.1016/s0140-6736(86)92212-9
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047. https://doi.org/10.1126/science.276.5321.2045
Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP (2013) Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep 14:389–394. https://doi.org/10.1038/embor.2013.15
Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H et al (2002) An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol 52:511–516. https://doi.org/10.1002/ana.10340
Porta S, Xu Y, Restrepo CR, Kwong LK, Zhang B, Brown HJ et al (2018) Patient-derived frontotemporal lobar degeneration brain extracts induce formation and spreading of TDP-43 pathology in vivo. Nat Commun 9:4220. https://doi.org/10.1038/s41467-018-06548-9
Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL et al (2013) A novel alpha-synuclein missense mutation in Parkinson disease. Neurology 80:1062–1064. https://doi.org/10.1212/WNL.0b013e31828727ba
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. https://doi.org/10.1126/science.6801762
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383. https://doi.org/10.1073/pnas.95.23.13363
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB et al (2015) Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci USA 112:E5308-5317. https://doi.org/10.1073/pnas.1514475112
Rasool CG, Selkoe DJ (1985) Sharing of specific antigens by degenerating neurons in Pick’s disease and Alzheimer’s disease. N Engl J Med 312:700–705. https://doi.org/10.1056/NEJM198503143121107
Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A et al (2014) Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75:351–362. https://doi.org/10.1002/ana.24066
Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. https://doi.org/10.1016/j.neuron.2011.09.010
Roberts GW (1988) Immunocytochemistry of neurofibrillary tangles in dementia pugilistica and Alzheimer’s disease: evidence for common genesis. Lancet 2:1456–1458. https://doi.org/10.1016/s0140-6736(88)90934-8
Rossi M, Candelise N, Baiardi S, Capellari S, Giannini G, Orru CD et al (2020) Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol 140:49–62. https://doi.org/10.1007/s00401-020-02160-8
Roy S, Wolman L (1969) Ultrastructural observations in Parkinsonism. J Pathol 99:39–44. https://doi.org/10.1002/path.1710990106
Rutherford NJ, Moore BD, Golde TE, Giasson BI (2014) Divergent effects of the H50Q and G51D SNCA mutations on the aggregation of alpha-synuclein. J Neurochem 131:859–867. https://doi.org/10.1111/jnc.12806
Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M et al (1998) Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4:1157–1165. https://doi.org/10.1038/2654
Saijo E, Ghetti B, Zanusso G, Oblak A, Furman JL, Diamond MI et al (2017) Ultrasensitive and selective detection of 3-repeat tau seeding activity in Pick disease brain and cerebrospinal fluid. Acta Neuropathol 133:751–765. https://doi.org/10.1007/s00401-017-1692-z
Saijo E, Metrick MA 2nd, Koga S, Parchi P, Litvan I, Spina S et al (2020) 4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathol 139:63–77. https://doi.org/10.1007/s00401-019-02080-2
Saito T, Mihira N, Matsuba Y, Sasaguri H, Hashimoto S, Narasimhan S et al (2019) Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J Biol Chem 294:12754–12765. https://doi.org/10.1074/jbc.RA119.009487
Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M et al (2003) Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol 62:644–654. https://doi.org/10.1093/jnen/62.6.644
Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y et al (2004) Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 63:911–918. https://doi.org/10.1093/jnen/63.9.911
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S et al (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287:3842–3849. https://doi.org/10.1074/jbc.M111.277061
Samuel F, Flavin WP, Iqbal S, Pacelli C, Sri Renganathan SD, Trudeau LE et al (2016) Effects of serine 129 phosphorylation on alpha-synuclein aggregation, membrane association, and internalization. J Biol Chem 291:4374–4385. https://doi.org/10.1074/jbc.M115.705095
Sano K, Atarashi R, Satoh K, Ishibashi D, Nakagaki T, Iwasaki Y et al (2018) Prion-like seeding of misfolded alpha-synuclein in the brains of dementia with Lewy body patients in RT-QUIC. Mol Neurobiol 55:3916–3930. https://doi.org/10.1007/s12035-017-0624-1
Schmid AW, Fauvet B, Moniatte M, Lashuel HA (2013) Alpha-synuclein post-translational modifications as potential biomarkers for Parkinson disease and other synucleinopathies. Mol Cell Proteom 12:3543–3558. https://doi.org/10.1074/mcp.R113.032730
Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B et al (2020) Structures of alpha-synuclein filaments from multiple system atrophy. Nature 585:464–469. https://doi.org/10.1038/s41586-020-2317-6
Scialo C, Tran TH, Salzano G, Novi G, Caponnetto C, Chio A et al (2020) TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun 2:fcaa142. https://doi.org/10.1093/braincomms/fcaa142
Seidler P, Boyer DR, Sawaya M, Ge P, Shin W, DeTure M et al (2020) CryoEM reveals how the small molecule EGCG binds to Alzheimer’s brain-derived tau fibrils and initiates fibril disaggregation. bioRxiv. https://doi.org/10.1101/2020.05.29.124537
Seidler PM, Boyer DR, Murray KA, Yang TP, Bentzel M, Sawaya MR et al (2019) Structure-based inhibitors halt prion-like seeding by Alzheimer’s disease-and tauopathy-derived brain tissue samples. J Biol Chem 294:16451–16464. https://doi.org/10.1074/jbc.RA119.009688
Selkoe DJ, Ihara Y, Salazar FJ (1982) Alzheimer’s disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science 215:1243–1245. https://doi.org/10.1126/science.6120571
Shahnawaz M, Mukherjee A, Pritzkow S, Mendez N, Rabadia P, Liu X et al (2020) Discriminating alpha-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578:273–277. https://doi.org/10.1038/s41586-020-1984-7
Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C et al (2017) Development of a biochemical diagnosis of Parkinson disease by detection of alpha-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 74:163–172. https://doi.org/10.1001/jamaneurol.2016.4547
Shi Y, Murzin AG, Falcon B, Epstein A, Machin J, Tempest P et al (2021) Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol 141:697–708. https://doi.org/10.1007/s00401-021-02294-3
Shi Y, Zhang W, Yang Y, Murzin AG, Falcon B, Kotecha A et al (2021) Structure-based classification of tauopathies. Nature 598:359–363. https://doi.org/10.1038/s41586-021-03911-7
Shimonaka S, Nonaka T, Suzuki G, Hisanaga S, Hasegawa M (2016) Templated aggregation of TAR DNA-binding protein of 43 kDa (TDP-43) by seeding with TDP-43 peptide fibrils. J Biol Chem 291:8896–8907. https://doi.org/10.1074/jbc.M115.713552
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J et al (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841. https://doi.org/10.1126/science.1090278
Smethurst P, Newcombe J, Troakes C, Simone R, Chen YR, Patani R et al (2016) In vitro prion-like behaviour of TDP-43 in ALS. Neurobiol Dis 96:236–247. https://doi.org/10.1016/j.nbd.2016.08.007
Smith MA, Harris PL, Sayre LM, Perry G (1997) Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA 94:9866–9868. https://doi.org/10.1073/pnas.94.18.9866
Snow AD, Mar H, Nochlin D, Sekiguchi RT, Kimata K, Koike Y et al (1990) Early accumulation of heparan sulfate in neurons and in the beta-amyloid protein-containing lesions of Alzheimer’s disease and Down’s syndrome. Am J Pathol 137:1253–1270
Solano SM, Miller DW, Augood SJ, Young AB, Penney JB Jr (2000) Expression of alpha-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: genes associated with familial Parkinson’s disease. Ann Neurol 47:201–210
Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M (1998) Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 251:205–208. https://doi.org/10.1016/s0304-3940(98)00504-7
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. https://doi.org/10.1073/pnas.95.11.6469
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 95:7737–7741. https://doi.org/10.1073/pnas.95.13.7737
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. https://doi.org/10.1038/42166
Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672. https://doi.org/10.1126/science.1154584
Suarez-Calvet M, Karikari TK, Ashton NJ, Lantero Rodriguez J, Mila-Aloma M, Gispert JD et al (2020) Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Abeta pathology are detected. EMBO Mol Med 12:e12921. https://doi.org/10.15252/emmm.202012921
Sun Y, Hou S, Zhao K, Long H, Liu Z, Gao J et al (2020) Cryo-EM structure of full-length alpha-synuclein amyloid fibril with Parkinson’s disease familial A53T mutation. Cell Res 30:360–362. https://doi.org/10.1038/s41422-020-0299-4
Sun Y, Long H, Xia W, Wang K, Zhang X, Sun B et al (2021) The hereditary mutation G51D unlocks a distinct fibril strain transmissible to wild-type alpha-synuclein. Nat Commun 12:6252. https://doi.org/10.1038/s41467-021-26433-2
Suzuki G, Imura S, Hosokawa M, Katsumata R, Nonaka T, Hisanaga SI et al (2020) alpha-synuclein strains that cause distinct pathologies differentially inhibit proteasome. Elife. https://doi.org/10.7554/eLife.56825
Takahashi T, Amano N, Hanihara T, Nagatomo H, Yagishita S, Itoh Y et al (1996) Corticobasal degeneration: widespread argentophilic threads and glia in addition to neurofibrillary tangles. Similarities of cytoskeletal abnormalities in corticobasal degeneration and progressive supranuclear palsy. J Neurol Sci 138:66–77. https://doi.org/10.1016/0022-510x(95)00347-5
Tanei ZI, Saito Y, Ito S, Matsubara T, Motoda A, Yamazaki M et al (2021) Lewy pathology of the esophagus correlates with the progression of Lewy body disease: a Japanese cohort study of autopsy cases. Acta Neuropathol 141:25–37. https://doi.org/10.1007/s00401-020-02233-8
Taniguchi-Watanabe S, Arai T, Kametani F, Nonaka T, Masuda-Suzukake M, Tarutani A et al (2016) Biochemical classification of tauopathies by immunoblot, protein sequence and mass spectrometric analyses of sarkosyl-insoluble and trypsin-resistant tau. Acta Neuropathol 131:267–280. https://doi.org/10.1007/s00401-015-1503-3
Tardivel M, Begard S, Bousset L, Dujardin S, Coens A, Melki R et al (2016) Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun 4:117. https://doi.org/10.1186/s40478-016-0386-4
Tarutani A, Arai T, Murayama S, Hisanaga SI, Hasegawa M (2018) Potent prion-like behaviors of pathogenic alpha-synuclein and evaluation of inactivation methods. Acta Neuropathol Commun 6:29. https://doi.org/10.1186/s40478-018-0532-2
Tarutani A, Miyata H, Nonaka T, Hasegawa K, Yoshida M, Saito Y et al (2021) Human tauopathy-derived tau strains determine the substrates recruited for templated amplification. Brain 144:2333–2348. https://doi.org/10.1093/brain/awab091
Tatsumi S, Uchihara T, Aiba I, Iwasaki Y, Mimuro M, Takahashi R et al (2014) Ultrastructural differences in pretangles between Alzheimer disease and corticobasal degeneration revealed by comparative light and electron microscopy. Acta Neuropathol Commun 2:161. https://doi.org/10.1186/s40478-014-0161-3
Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R et al (1996) Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082. https://doi.org/10.1126/science.274.5295.2079
Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M et al (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556. https://doi.org/10.1093/jnen/61.6.547
Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M et al (2011) Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci 14:452–458. https://doi.org/10.1038/nn.2778
Tracy TE, Madero-Perez J, Swaney DL, Chang TS, Moritz M, Konrad C et al (2022) Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration. Cell 185(712–728):e714. https://doi.org/10.1016/j.cell.2021.12.041
Trinkaus VA, Riera-Tur I, Martinez-Sanchez A, Bauerlein FJB, Guo Q, Arzberger T et al (2021) In situ architecture of neuronal alpha-synuclein inclusions. Nat Commun 12:2110. https://doi.org/10.1038/s41467-021-22108-0
Tsuji H, Arai T, Kametani F, Nonaka T, Yamashita M, Suzukake M et al (2012) Molecular analysis and biochemical classification of TDP-43 proteinopathy. Brain 135:3380–3391. https://doi.org/10.1093/brain/aws230
Uchihara T, Nakamura A, Yamazaki M, Mori O (2001) Evolution from pretangle neurons to neurofibrillary tangles monitored by thiazin red combined with Gallyas method and double immunofluorescence. Acta Neuropathol 101:535–539. https://doi.org/10.1007/s004010000306
Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M et al (1993) Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA 90:11282–11286. https://doi.org/10.1073/pnas.90.23.11282
Urrea L, Segura-Feliu M, Masuda-Suzukake M, Hervera A, Pedraz L, Garcia Aznar JM et al (2018) Involvement of cellular prion protein in alpha-synuclein transport in neurons. Mol Neurobiol 55:1847–1860. https://doi.org/10.1007/s12035-017-0451-4
Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564. https://doi.org/10.1097/NEN.0b013e31817713b5
Vandermeeren M, Mercken M, Vanmechelen E, Six J, van de Voorde A, Martin JJ et al (1993) Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem 61:1828–1834. https://doi.org/10.1111/j.1471-4159.1993.tb09823.x
Varani L, Hasegawa M, Spillantini MG, Smith MJ, Murrell JR, Ghetti B et al (1999) Structure of tau exon 10 splicing regulatory element RNA and destabilization by mutations of frontotemporal dementia and parkinsonism linked to chromosome 17. Proc Natl Acad Sci USA 96:8229–8234. https://doi.org/10.1073/pnas.96.14.8229
Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M et al (1998) Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol 96:445–452. https://doi.org/10.1007/s004010050918
Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H (1998) Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 249:180–182. https://doi.org/10.1016/s0304-3940(98)00407-8
Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K et al (2017) The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12:5. https://doi.org/10.1186/s13024-016-0143-y
Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM et al (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA 110:19555–19560. https://doi.org/10.1073/pnas.1318268110
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 72:1858–1862. https://doi.org/10.1073/pnas.72.5.1858
Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P et al (2020) Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell 183(1699–1713):e1613. https://doi.org/10.1016/j.cell.2020.10.029
Whitehouse PJ, Hedreen JC, White CL 3rd, Price DL (1983) Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol 13:243–248. https://doi.org/10.1002/ana.410130304
Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ et al (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130:1566–1576. https://doi.org/10.1093/brain/awm104
Wilson DM, Binder LI (1997) Free fatty acids stimulate the polymerization of tau and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer’s disease. Am J Pathol 150:2181–2195
Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, Lee VM (2008) Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem 283:13302–13309. https://doi.org/10.1074/jbc.M800342200
Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA (1988) Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85:4884–4888. https://doi.org/10.1073/pnas.85.13.4884
Wischik CM, Novak M, Thogersen HC, Edwards PC, Runswick MJ, Jakes R et al (1988) Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85:4506–4510. https://doi.org/10.1073/pnas.85.12.4506
Woerman AL, Aoyagi A, Patel S, Kazmi SA, Lobach I, Grinberg LT et al (2016) Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci USA 113:E8187–E8196. https://doi.org/10.1073/pnas.1616344113
Woerman AL, Stohr J, Aoyagi A, Rampersaud R, Krejciova Z, Watts JC et al (2015) Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci USA 112:E4949-4958. https://doi.org/10.1073/pnas.1513426112
Wong CW, Quaranta V, Glenner GG (1985) Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci USA 82:8729–8732. https://doi.org/10.1073/pnas.82.24.8729
Yamada T, McGeer PL, McGeer EG (1992) Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett 135:99–102. https://doi.org/10.1016/0304-3940(92)90145-w
Yamasaki TR, Holmes BB, Furman JL, Dhavale DD, Su BW, Song ES et al (2019) Parkinson’s disease and multiple system atrophy have distinct alpha-synuclein seed characteristics. J Biol Chem 294:1045–1058. https://doi.org/10.1074/jbc.RA118.004471
Yoshimoto M, Iwai A, Kang D, Otero DA, Xia Y, Saitoh T (1995) NACP, the precursor protein of the non-amyloid beta/A4 protein (A beta) component of Alzheimer disease amyloid, binds A beta and stimulates A beta aggregation. Proc Natl Acad Sci USA 92:9141–9145. https://doi.org/10.1073/pnas.92.20.9141
Yoshino H, Hirano M, Stoessl AJ, Imamichi Y, Ikeda A, Li Y et al (2017) Homozygous alpha-synuclein p. A53V in familial Parkinson’s disease. Neurobiol Aging 57:248 e247-248 e212. https://doi.org/10.1016/j.neurobiolaging.2017.05.022
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173. https://doi.org/10.1002/ana.10795
Zhang W, Falcon B, Murzin AG, Fan J, Crowther RA, Goedert M et al (2019) Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer’s and Pick’s diseases. Elife. https://doi.org/10.7554/eLife.43584
Zhang W, Tarutani A, Newell KL, Murzin AG, Matsubara T, Falcon B et al (2020) Novel tau filament fold in corticobasal degeneration. Nature 580:283–287. https://doi.org/10.1038/s41586-020-2043-0
Zhao K, Li Y, Liu Z, Long H, Zhao C, Luo F et al (2020) Parkinson’s disease associated mutation E46K of alpha-synuclein triggers the formation of a distinct fibril structure. Nat Commun 11:2643. https://doi.org/10.1038/s41467-020-16386-3
Acknowledgements
We are grateful to the patients and their families for making tissues available for research. Tissue samples used for immunohistochemistry, immunoelectron microscopy and immunoblotting were supplied by the Japan Brain Banks at the Tokyo Metropolitan Geriatric Hospital and Institute of gerontology, Aichi Medical University, National Center of Neurology and Psychiatry, Fukushimura Hospital and Tottori University, and the Manchester Brain Bank. Molecular graphics were created with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311 [230]. This work was supported by Japan Science and Technology Agency (JST) CREST Grant Number JPMJCR18H3 (to MH), The Japan Agency for Medical Research and Development (AMED) under Grant Number JP18ek0109391 and JP18dm0207019 (to M.H.). The Manchester Brain Bank is part of the Brains for Dementia Research programme, jointly funded by Alzheimer’s Research UK and Alzheimer’s Society.
Author information
Authors and Affiliations
Contributions
AT and MH conceptualized the manuscript. AT wrote the original manuscript and prepared the figures. AT, MH and ACR performed immunohistochemistry, immunoelectron microscopy and immunoblotting for the figures. SM, MY, DMAM, ACR, YS, KH, YH, HA and TA collected patients’ brain samples and performed neuropathological diagnosis. MH critically revised the manuscript. All authors reviewed and agreed on the content of the manuscript before submission.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarutani, A., Adachi, T., Akatsu, H. et al. Ultrastructural and biochemical classification of pathogenic tau, α-synuclein and TDP-43. Acta Neuropathol 143, 613–640 (2022). https://doi.org/10.1007/s00401-022-02426-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02426-3