Abstract
Background and Objective
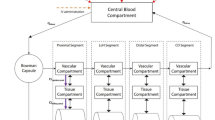
The renal excretion of drugs via organic anion transporters 1 and 3 (OAT1/3) is significantly decreased in patients with renal impairment. This study uses physiologically based pharmacokinetic models to quantify the reduction in OAT1/3-mediated secretion of drugs throughout varying stages of chronic kidney disease.
Methods
Physiologically based pharmacokinetic models were constructed for four OAT1/3 substrates in healthy individuals: acyclovir, meropenem, furosemide, and ciprofloxacin. Observed data from drug–drug interaction studies with probenecid, a potent OAT1/3 inhibitor, were used to parameterize the contribution of OAT1/3 to the renal elimination of each drug. The models were then translated to patients with chronic kidney disease by accounting for changes in glomerular filtration rate, kidney volume, renal blood flow, plasma protein binding, and hematocrit. Additionally, a relationship was derived between the estimated glomerular filtration rate and the reduction in OAT1/3-mediated secretion of drugs based on the renal extraction ratios of ƿ-aminohippuric acid in patients with varying degrees of renal impairment. The relationship was evaluated in silico by evaluating the predictive performance of each final model in describing the pharmacokinetics (PK) of drugs across stages of chronic kidney disease.
Results
OAT1/3-mediated renal excretion of drugs was found to be decreased by 27–49%, 50–68%, and 70–96% in stage 3, stage 4, and stage 5 of chronic kidney disease, respectively. In support of the parameterization, physiologically based pharmacokinetic models of four OAT1/3 substrates were able to adequately characterize the PK in patients with different degrees of renal impairment. Total exposure after intravenous administration was predicted within a 1.5-fold error and 85% of the observed data points fell within a 1.5-fold prediction error. The models modestly under-predicted plasma concentrations in patients with end-stage renal disease undergoing intermittent hemodialysis. However, results should be interpreted with caution because of the limited number of molecules analyzed and the sparse sampling in observed chronic kidney disease pharmacokinetic studies.
Conclusions
A quantitative understanding of the reduction in OAT1/3-mediated excretion of drugs in differing stages of renal impairment will contribute to better predictive accuracy for physiologically based pharmacokinetic models in drug development, assisting with clinical trial planning and potentially sparing this population from unnecessary toxic exposures.








Similar content being viewed by others
References
Purcell CA, Abebe M, Adebayo OM, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–33. https://doi.org/10.1016/S0140-6736(20)30045-3.
KDIGO (Kidney Disease: Improving Global Outcomes). Acute kidney injury. 2012. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. Accessed 18 Mar 2022.
Manley HJ, Cannella CA, Bailie GR, St Peter WL. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney Dis. 2005;46(4):669–80. https://doi.org/10.1053/j.ajkd.2005.07.001.
Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–29. https://doi.org/10.1146/annurev-pharmtox-011112-140317.
Torres AM, Dnyanmote AV, Granados JC, Nigam SK. Renal and non-renal response of ABC and SLC transporters in chronic kidney disease. Expert Opin Drug Metab Toxicol. 2021;17(5):515–42. https://doi.org/10.1080/17425255.2021.1899159.
Cheung KWK, Hsueh C, Zhao P, et al. The effect of uremic solutes on the organic cation transporter 2. J Pharm Sci. 2017;106(9):2551–7. https://doi.org/10.1016/j.xphs.2017.04.076.
Malik PRV, Yeung CHT, Ismaeil S, Advani U, Djie S, Edginton AN. A physiological approach to pharmacokinetics in chronic kidney disease. J Clin Pharmacol. 2020;60(S1):S52-62. https://doi.org/10.1002/jcph.1713.
Droździk M, Oswald S, Droździk A. Impact of kidney dysfunction on hepatic and intestinal drug transporters. Biomed Pharmacother. 2021;143: 112125. https://doi.org/10.1016/j.biopha.2021.112125.
Masereeuw R, Mutsaers HAM, Toyohara T, et al. The kidney and uremic toxin removal: glomerulus or tubule? Semin Nephrol. 2014;34(2):191–208. https://doi.org/10.1016/j.semnephrol.2014.02.010.
Hsueh C, Yoshida K, Zhao P, et al. Identification and quantitative assessment of uremic solutes as inhibitors of renal organic anion transporters, OAT1 and OAT3. Mol Pharm. 2016;13(9):3130–40. https://doi.org/10.1021/acs.molpharmaceut.6b00332.
Hsueh C, Hsu V, Zhao P, Zhang L, Giacomini KM, Huang S. PBPK modeling of the effect of reduced kidney function on the pharmacokinetics of drugs excreted renally by organic anion transporters. Clin Pharmacol Ther. 2018;103(3):485–92. https://doi.org/10.1002/cpt.750.
Willmann S, Höhn K, Edginton A, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34(3):401–31. https://doi.org/10.1007/s10928-007-9053-5.
Schlender J, Meyer M, Thelen K, et al. Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin Pharmacokinet. 2016;55(12):1573–89. https://doi.org/10.1007/s40262-016-0422-3.
Schlender J, Teutonico D, Coboeken K, et al. A physiologically-based pharmacokinetic model to describe ciprofloxacin pharmacokinetics over the entire span of life. Clin Pharmacokinet. 2018;57(12):1613–34. https://doi.org/10.1007/s40262-018-0661-6.
Britz H, Hanke N, Taub ME, et al. Physiologically based pharmacokinetic models of probenecid and furosemide to predict transporter mediated drug-drug interactions. Pharm Res. 2020;37(12):250. https://doi.org/10.1007/s11095-020-02964-z.
Mouton JW, van den Anker JN. Meropenem clinical pharmacokinetics. Clin Pharmacokinet. 1995;28(4):275–86. https://doi.org/10.2165/00003088-199528040-00002.
Merrem (meropenem). Prescribing information. Boucherville: Sandoz Canada Inc.; 2017.
Arnal J, Gonzalez-Alvarez I, Bermejo M, et al. Biowaiver monographs for immediate release solid oral dosage forms: aciclovir. J Pharm Sci. 2008;97(12):5061–73. https://doi.org/10.1002/jps.21392.
DrugBank. Meropenem. https://go.drugbank.com/drugs/DB00760. Accessed 18 Mar 2022.
Jaehde U, Sörgel F, Reiter A, Sigl G, Naber KG, Schunack W. Effect of probenecid on the distribution and elimination of ciprofloxacin in humans. Clin Pharmacol Ther. 1995;58(5):532–41.
Bax RP, Bastain W, Featherstone A, Wilkinson DM, Hutchison M, Haworth SJ. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989;24(Suppl A):311–20.
de Miranda P, Good SS, Laskin OL, Krasny HC, Connor JD, Lietman PS. Disposition of intravenous radioactive acyclovir. Clin Pharmacol Ther. 1981;30(5):662–72.
Momper JD, Tsunoda SM, Ma JD. Evaluation of proposed in vivo probe substrates and inhibitors for phenotyping transporter activity in humans. J Clin Pharmacol. 2016;56(Suppl. 7):82. https://doi.org/10.1002/jcph.736.
Shibayama T, Sugiyama D, Kamiyama E, Tokui T, Hirota T, Ikeda T. Characterization of CS-023 (RO4908463), a novel parenteral carbapenem antibiotic, and meropenem as substrates of human renal transporters. Drug Metab Pharmacokinet. 2007;22(1):41–7. https://doi.org/10.2133/dmpk.22.41.
Ivanyuk A, Livio F, Biollaz J, Buclin T. Renal drug transporters and drug interactions. Clin Pharmacokinet. 2017;56(8):825–92. https://doi.org/10.1007/s40262-017-0506-8.
Scotcher D, Billington S, Brown J, et al. Microsomal and cytosolic scaling factors in dog and human kidney cortex and application for in vitro-in vivo extrapolation of renal metabolic clearance. Drug Metab Dispos. 2017;45(5):556–8. https://doi.org/10.1124/dmd.117.075242.
Prasad B, Johnson K, Billington S, et al. Abundance of drug transporters in the human kidney cortex as quantified by quantitative targeted proteomics. Drug Metab Dispos. 2016;44(12):1920–4. https://doi.org/10.1124/dmd.116.072066.
Uchino H, Tamai I, Yabuuchi H, et al. Faropenem transport across the renal epithelial luminal membrane via inorganic phosphate transporter Npt1. Antimicrob Agents Chemother. 2000;44(3):574–7.
Willmann S, Lippert J, Schmitt W. From physicochemistry to absorption and distribution: predictive mechanistic modelling and computational tools. Expert Opin Drug Metab Toxicol. 2005;1(1):159–68. https://doi.org/10.1517/17425255.1.1.159.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76. https://doi.org/10.1002/jps.20322.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57. https://doi.org/10.1002/jps.20502.
Hsu V, de Vieira LT, Manuela ZP, et al. Towards quantitation of the effects of renal impairment and probenecid inhibition on kidney uptake and efflux transporters, using physiologically based pharmacokinetic modelling and simulations. Clin Pharmacokinet. 2014;53(3):283–93. https://doi.org/10.1007/s40262-013-0117-y.
Doki K, Neuhoff S, Rostami-Hodjegan A, Homma M. Assessing potential drug–drug interactions between dabigatran etexilate and P-glycoprotein inhibitor in renal impairment populations using physiologically based pharmacokinetic modeling. CPT Pharmacomet Syst Pharmacol. 2019;8(2):118–26.
Tsamandouras N, Rostami-Hodjegan A, Aarons L. Combining the ‘bottom up’ and ‘top down’ approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2015;79(1):48–55.
Sayama H, Takubo H, Komura H, Kogayu M, Iwaki M. Application of a physiologically based pharmacokinetic model informed by a top-down approach for the prediction of pharmacokinetics in chronic kidney disease patients. AAPS J. 2014;16(5):1018–28. https://doi.org/10.1208/s12248-014-9626-3.
Basit A, Radi Z, Vaidya VS, Karasu M, Prasad B. Kidney cortical transporter expression across species using quantitative proteomics. Drug Metab Dispos. 2019;47(8):802–8. https://doi.org/10.1124/dmd.119.086579.
Mahmood I, Tegenge MA. A comparative study between allometric scaling and physiologically based pharmacokinetic modeling for the prediction of drug clearance from neonates to adolescents. J Clin Pharmacol. 2019;59(2):189–97. https://doi.org/10.1002/jcph.1310.
Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M. The renal clearances of substituted hippuric acid derivatives and other aromatic acids in dog and man. J Clin Investig. 1945;24(3):388–404. https://doi.org/10.1172/JCI101618.
Nigam SK, Bush KT, Martovetsky G, et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev. 2015;95(1):83–123. https://doi.org/10.1152/physrev.00025.2013.
Ullrich KJ, Rumrich G. Renal transport mechanisms for xenobiotics: chemicals and drugs. Clin Investig. 1993;71(10):843–8. https://doi.org/10.1007/BF00190334.
Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618(2):185–93. https://doi.org/10.1016/j.bbamem.2003.08.015.
Battilana C, Zhang HP, Olshen RA, Wexler L, Myers BD. PAH extraction and estimation of plasma flow in diseased human kidneys. Am J Physiol. 1991;261(4 Pt 2):726. https://doi.org/10.1152/ajprenal.1991.261.4.F726.
Bergstrom J, Bucht H, Ek J, Josephson B, Sundell H, Werko L. The renal extraction of para-aminohippurate in normal persons and in patients with diseased kidneys. Scand J Clin Lab Investig. 1959;11:361–75. https://doi.org/10.3109/00365515909060466.
Bradley SE, Bradley GP, Tyson CJ, Curry JJ, Blake WD. Renal function in renal diseases. Am J Med. 1950;9(6):766–98. https://doi.org/10.1016/0002-9343(50)90292-0.
Brodwall EK. Renal extraction of PAH in renal disease. Scand J Clin Lab Investig. 1964;16(1):12–20. https://doi.org/10.3109/00365516409060477.
Josephson B, Bucht H, Ek J, Werko L. Renal extraction, its depression, and the tubular storage of p-aminohippuric acid (PAH) in the healthy and in the diseased human kidney. Scand J Clin Lab Investig. 1952;4(1):1–14. https://doi.org/10.3109/00365515209060626.
Cargill WH. The measurement of glomerular and tubular plasma flow in the normal and diseased human kidney. J Clin Investig. 1949;28(3):533–8.
Zhang Y, Zhang L, Abraham S, et al. Assessment of the impact of renal impairment on systemic exposure of new molecular entities: evaluation of recent new drug applications. Clin Pharmacol Ther. 2009;85(3):305–11. https://doi.org/10.1038/clpt.2008.208.
Zhang L, Xu N, Xiao S, et al. Regulatory perspectives on designing pharmacokinetic studies and optimizing labeling recommendations for patients with chronic kidney disease. J Clin Pharmacol. 2012;52(1 Suppl.):79S-90S. https://doi.org/10.1177/0091270011415410.
Yoshida K, Sun B, Zhang L, et al. Systematic and quantitative assessment of the effect of chronic kidney disease on CYP2D6 and CYP3A4/5. Clin Pharmacol Ther. 2016;100(1):75–87. https://doi.org/10.1002/cpt.337.
Laskin OL, de Miranda P, King DH, et al. Effects of probenecid on the pharmacokinetics and elimination of acyclovir in humans. Antimicrob Agents Chemother. 1982;21(5):804–7.
Wiebe ST, Giessmann T, Hohl K, et al. Validation of a drug transporter probe cocktail using the prototypical inhibitors rifampin, probenecid, verapamil, and cimetidine. Clin Pharmacokinet. 2020;59(12):1627–39. https://doi.org/10.1007/s40262-020-00907-w.
Chimata M, Nagase M, Suzuki Y, Shimomura M, Kakuta S. Pharmacokinetics of meropenem in patients with various degrees of renal function, including patients with end-stage renal disease. Antimicrob Agents Chemother. 1993;37(2):229–33.
Drusano GL, Weir M, Forrest A, Plaisance K, Emm T, Standiford HC. Pharmacokinetics of intravenously administered ciprofloxacin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1987;31(6):860–4. https://doi.org/10.1128/AAC.31.6.860.
Shah A, Lettieri J, Blum R, Millikin S, Sica D, Heller AH. Pharmacokinetics of intravenous ciprofloxacin in normal and renally impaired subjects. J Antimicrob Chemother. 1996;38(1):103–16. https://doi.org/10.1093/jac/38.1.103.
Christensson BA, Nilsson-Ehle I, Hutchison M, Haworth SJ, Oqvist B, Norrby SR. Pharmacokinetics of meropenem in subjects with various degrees of renal impairment. Antimicrob Agents Chemother. 1992;36(7):1532–7. https://doi.org/10.1128/AAC.36.7.1532.
Leroy A, Fillastre JP, Etienne I, Borsa-Lebás F, Humbert G. Pharmacokinetics of meropenem in subjects with renal insufficiency. Eur J Clin Pharmacol. 1992;42(5):535–8. https://doi.org/10.1007/BF00314864.
Gladziwa U, Böhm R, Klotz U, et al. Pharmacokinetics of furosemide in patients with chronic renal failure. Drug Investig. 1993;6:137–43. https://doi.org/10.1007/BF03259733.
Rane A, Villeneuve JP, Stone WJ, Nies AS, Wilkinson GR, Branch RA. Plasma binding and disposition of furosemide in the nephrotic syndrome and in uremia. Clin Pharmacol Ther. 1978;24(2):199–207. https://doi.org/10.1002/cpt1978242199.
Laskin OL, Longstreth JA, Whelton A, et al. Acyclovir kinetics in end-stage renal disease. Clin Pharmacol Ther. 1982;31(5):594–601. https://doi.org/10.1038/clpt.1982.83.
Bush KT, Singh P, Nigam SK. Gut-derived uremic toxin handling in vivo requires OAT-mediated tubular secretion in chronic kidney disease. JCI Insight. 2020;9(5): e133817. https://doi.org/10.1172/jci.insight.133817.
Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharmacol. 2011;4(2):261–74. https://doi.org/10.1586/ecp.10.143.
Tan M, Yoshida K, Zhao P, et al. Effect of chronic kidney disease on nonrenal elimination pathways: a systematic assessment of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and OATP. Clin Pharmacol Ther. 2018;103(5):854–67. https://doi.org/10.1002/cpt.807.
Tan M, Zhao P, Zhang L, et al. Use of physiologically based pharmacokinetic modeling to evaluate the effect of chronic kidney disease on the disposition of hepatic CYP2C8 and OATP1B drug substrates. Clin Pharmacol Ther. 2019;105(3):719–29. https://doi.org/10.1002/cpt.1205.
Follman KE, Morris ME. Prediction of the effects of renal impairment on clearance for organic cation drugs that undergo renal secretion: a simulation-based study. Drug Metab Dispos. 2018;46(5):758–69. https://doi.org/10.1124/dmd.117.079558.
Bricker NS. On the meaning of the intact nephron hypothesis. Am J Med. 1969;46(1):1–11. https://doi.org/10.1016/0002-9343(69)90053-9.
Dettli L. Drug dosage in renal disease. Clin Pharmacokinet. 1976;1(2):126–34. https://doi.org/10.2165/00003088-197601020-00004.
Chapron A, Shen DD, Kestenbaum BR, Robinson-Cohen C, Himmelfarb J, Yeung CK. Does secretory clearance follow glomerular filtration rate in chronic kidney diseases? Reconsidering the intact nephron hypothesis. Clin Transl Sci. 2017;10(5):395–403. https://doi.org/10.1111/cts.12481.
Momper JD, Yang J, Gockenbach M, Vaida F, Nigam SK. Dynamics of organic anion transporter-mediated tubular secretion during postnatal human kidney development and maturation. Clin J Am Soc Nephrol. 2019;14(4):540–8. https://doi.org/10.2215/CJN.10350818.
Toto RD. Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens. 1995;4(6):505–9. https://doi.org/10.1097/00041552-199511000-00009.
Dowling TC, Frye RF, Fraley DS, Matzke GR. Characterization of tubular functional capacity in humans using para-aminohippurate and famotidine. Kidney Int. 2001;59(1):295–303. https://doi.org/10.1046/j.1523-1755.2001.00491.x.
Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277(30):26934–43. https://doi.org/10.1074/jbc.M203803200.
Eraly SA, Vallon V, Vaughn DA, et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem. 2006;281(8):5072–83. https://doi.org/10.1074/jbc.M508050200.
Rizwan AN, Krick W, Burckhardt G. The chloride dependence of the human organic anion transporter 1 (hOAT1) is blunted by mutation of a single amino acid. J Biol Chem. 2007;282(18):13402–9. https://doi.org/10.1074/jbc.M609849200.
Burckhardt G, Bahn A, Wolff NA. Molecular physiology of renal p-aminohippurate secretion. News Physiol Sci. 2001;16:114–8. https://doi.org/10.1152/physiologyonline.2001.16.3.114.
Schumann L, Wüstenberg PW. An improved method to determine renal function using inulin and p-aminohippurate (PAH) steady-state kinetic modeling. Clin Nephrol. 1990;33(1):35–40.
Hanke N, Frechen S, Moj D, et al. PBPK models for CYP3A4 and p-gp DDI prediction: a modeling network of rifampicin, itraconazole, clarithromycin, midazolam, alfentanil, and digoxin. CPT Pharmacomet Syst Pharmacol. 2018;7(10):647–59. https://doi.org/10.1002/psp4.12343.
Center for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review: olumiant. Published December 2017. Baricitinib. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207924Orig1s000ClinPharmR.pdf. Accessed Sep 2021.
Posada MM, Bacon JA, Schneck KB, et al. Prediction of renal transporter mediated drug–drug interactions for pemetrexed using physiologically based pharmacokinetic modeling. Drug Metab Dispos. 2015;43(3):325–34. https://doi.org/10.1124/dmd.114.059618.
Salerno SN, Carreño FO, Edginton AN, Cohen-Wolkowiez M, Gonzalez D. Leveraging physiologically based pharmacokinetic modeling and experimental data to guide dosing modification of CYP3A-mediated drug-drug interactions in the pediatric population. Drug Metab Dispos. 2021;49(9):844–55. https://doi.org/10.1124/dmd.120.000318.
Nolin TD, Frye RF, Matzke GR. Hepatic drug metabolism and transport in patients with kidney disease. Am J Kidney Dis. 2003;42(5):906–25. https://doi.org/10.1016/j.ajkd.2003.07.019.
Decker BS, O’Neill KD, Chambers MA, et al. Hemodialysis does not alter in vitro hepatic CYP3A4 and CYP2D6 metabolic activity in uremic serum. Clin Pharmacol. 2013;5:193–9. https://doi.org/10.2147/CPAA.S54381.
Hirako M, Kamiya T, Misu N, et al. Impaired gastric motility and its relationship to gastrointestinal symptoms in patients with chronic renal failure. J Gastroenterol. 2005;40(12):1116–22. https://doi.org/10.1007/s00535-005-1709-6.
Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junctions. Am J Nephrol. 2013;38(2):99–103. https://doi.org/10.1159/000353764.
Shitara Y, Maeda K, Ikejiri K, Yoshida K, Horie T, Sugiyama Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos. 2013;34(1):45–78. https://doi.org/10.1002/bdd.1823.
Nolin TD, Frye RF, Le P, et al. ESRD impairs nonrenal clearance of fexodenadine but not midazolam. J Am Soc Nephrol. 2009;20(10):2269–76. https://doi.org/10.1681/ASN.2009010082.
Thomson BKA, Nolin TD, Velenosi TJ, et al. Effect of CKD and dialysis modality on exposure to drugs cleared by nonrenal mechanisms. Am J Kidney Dis. 2015;65(4):574–82. https://doi.org/10.1053/j.ajkd.2014.09.015.
Naud J, Michaud J, Beauchemin S, et al. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos. 2011;39(8):1363–9. https://doi.org/10.1124/dmd.111.039115.
Torres AM, Laughlin MM, Muller A, Brandoni A, Anzai N, Endou H. Altered renal elimination of organic anions in rats with chronic renal failure. Biochim Biophys Acta. 2005;1740(1):29–37. https://doi.org/10.1016/j.bbadis.2005.03.002.
Deguchi T, Takemoto M, Uehara N, Lindup WE, Suenaga A, Otagiri M. Renal clearance of endogenous hippurate correlates with expression levels of renal organic anion transporters in uremic rats. J Pharmacol Exp Ther. 2005;314(2):932–8. https://doi.org/10.1124/jpet.105.085613.
Nishihara K, Masuda S, Ji L, Katsura T, Inui KI. Pharmacokinetic significance of luminal multidrug and toxin extrusion 1 in chronic renal failure rats. Biochem Pharmacol. 2007;73(9):1482–90. https://doi.org/10.1016/j.bcp.2006.12.034.
Xu G, Bhatnagar V, Wen B, Hamilton BA, Early SA, Nigam SK. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)]. Kidney Int. 2005;68(4):1491–9.
Stephens JC, Schneider JA, Tanguay DA, et al. Haplotype variation and linkage disequilibrium in 313 human genes. Science. 2001;293(5529):489–93.
Marzolini C, Tirona RG, Kim RB. Pharmacogenomics of the OATP and OAT families. Pharmacogenomics. 2004;5(3):273–82.
Nishizato Y, Ieiri I, Suzuki H, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences of pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73(6):554–65.
Lozano E, Briz O, Macias RIR, et al. Genetic heterogeneity of SLC22 family of transporters in drug disposition. J Pers Med. 2018;8(2):14. https://doi.org/10.3390/jpm8020014.
Wagner C, Zhao P, Pan Y, et al. Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: report of an FDA public workshop on PBPK. CPT Pharmacomet Syst Pharmacol. 2015;4(4):226–30. https://doi.org/10.1002/psp4.33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received for the preparation of this article.
Conflicts of interest
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
SD, PM, and AE conceptualized the idea and topic of this article. SD and PM contributed to a healthy adult PBPK model construction of the investigative drugs analyzed within this article. SD conducted the CKD-PBPK simulations and analyzed the results. DH was instrumental in the development and finalization of the OAT parameterization function used throughout the CKD-PBPK simulations. Contributions from PM were final as of 11 June, 2021. Preparation of the final manuscript was conducted by SD. The final version of manuscript was reviewed by all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dubinsky, S., Malik, P., Hajducek, D.M. et al. Determining the Effects of Chronic Kidney Disease on Organic Anion Transporter1/3 Activity Through Physiologically Based Pharmacokinetic Modeling. Clin Pharmacokinet 61, 997–1012 (2022). https://doi.org/10.1007/s40262-022-01121-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01121-6