Abstract
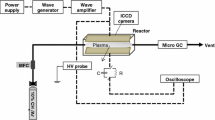
Plasma catalysis is a promising approach to further enhance the conversion of methane into value-added products such as methanol. In this work, the mechanisms enabling the conversion of methane to CO, CO2 and methanol enabled by plasma-enhanced catalysis were investigated. A catalyst reactor was incorporated downstream of the plasma jet to enable the separation between plasma generation and the catalyst bed. An enhancement in CH3OH and CO2 production was observed for the shortest distance between the plasma and catalyst compared to the plasma-only case. Plasma-enabled gas heating was shown not to be responsible for the observed synergy while a gas temperature increase as low as 30–40 K significantly impacted desorption rates of CH3OH/C2H5OH on alumina particles. Correlations between molecular beam mass spectrometry (MBMS) measurements at the inlet and outlet of the catalytic reactor suggest that the observed synergistic effect was caused by radical species most likely the CH3O2 radical. This study shows that surface reactions induced by radicals such as alkylperoxy radicals might play an important role in surface reactions in plasma-catalysis.











Similar content being viewed by others
References
Ravi M, Ranocchiari M, van Bokhoven JA (2017) The direct catalytic oxidation of methane to methanol-a critical assessment. Angew Chemie Int Ed 56(52):16464–16483
Joghee P, Malik JN, Pylypenko S, O’Hayre R (2015) A review on direct methanol fuel cells–in the perspective of energy and sustainability. MRS Energy Sustain 2(1):3
Larkin DW, Lobban LL, Mallinson RG (2001) The direct partial oxidation of methane to organic oxygenates using a dielectric barrier discharge reactor as a catalytic reactor analog. Catal Today 71(1–2):199–210
Bogaerts A, Tu X, Whitehead JC, Centi G, Lefferts L, Guaitella O, Azzolina-Jury F, Kim H-H, Murphy AB, Schneider WF et al (2020) The 2020 plasma catalysis roadmap. J Phys D Appl Phys 53(44):443001
Nozaki T, Okazaki K (2013) Non-thermal plasma catalysis of methane: principles, energy efficiency, and applications. Catal Today 211:29–38
Kim J, Go DB, Hicks JC (2017) Synergistic effects of plasma-catalyst interactions for CH 4 activation. Phys Chem Chem Phys 19(20):13010–13021
Kim J, Abbott MS, Go DB, Hicks JC (2016) Enhancing C-H bond activation of methane via temperature-controlled. Catalyst-Plasma Interactions ACS Energy Lett 1(1):94–99
Astafan A, Batiot-Dupeyrat C, Pinard L (2019) Mechanism and kinetic of coke oxidation by nonthermal plasma in fixed-bed dielectric barrier reactor. J Phys Chem C 123(14):9168–9175
Chawdhury P, Wang Y, Ray D, Mathieu S, Wang N, Harding J, Bin F, Tu X, Subrahmanyam C (2021) A promising plasma-catalytic approach towards single-step methane conversion to oxygenates at room temperature. Appl Catal B Environ 284:119735
De Bie C, Van Dijk J, Bogaerts A (2015) The dominant pathways for the conversion of methane into oxygenates and syngas in an atmospheric pressure dielectric barrier discharge. J Phys Chem C 119(39):22331–22350
Yi Y, Li S, Cui Z, Hao Y, Zhang Y, Wang L, Liu P, Tu X, Xu X, Guo H et al (2021) Selective oxidation of CH4 to CH3OH through plasma catalysis: insights from catalyst characterization and chemical kinetics modelling. Appl Catal B Environ 296:120384
Lustemberg PG, Palomino RM, Gutiérrez RA, Grinter DC, Vorokhta M, Liu Z, Ramírez PJ, Matolín V, Ganduglia-Pirovano MV, Senanayake SD et al (2018) Direct conversion of methane to methanol on ni-ceria surfaces: metal-support interactions and water-enabled catalytic conversion by site blocking. J Am Chem Soc 140(24):7681–7687
Li Y, Jiang J, Hinshelwood M, Zhang S, Bruggeman P, Oehrlein GS (2021) Characterization of Plasma Catalytic Decomposition of Methane: Role of Atomic O and Reaction Mechanism. J Phys D Appl Phys. https://doi.org/10.1088/1361-6463/ac4728
Hofmann S, van Gessel AFH, Verreycken T, Bruggeman P (2011) Power dissipation, gas temperatures and electron densities of cold atmospheric pressure helium and argon RF plasma Jets. Plasma Sources Sci Technol 20(6):065010
Jiang J, Luo Y, Moldgy A, ArandaGonzalvo Y, Bruggeman PJ (2020) Absolute spatially and time-resolved O, O3, and air densities in the effluent of a modulated RF-driven atmospheric pressure plasma jet obtained by molecular beam mass spectrometry. Plasma Process Polym 17(6):1900163
Baiocchi FA, Wetzel RC, Freund RS (1984) Electron-impact ionization and dissociative ionization of the CD3 and CD2 free radicals. Phys Rev Lett 53(8):771–774
Itikawa Y, Ichimura A, Onda K, Sakimoto K, Takayanagi K, Hatano Y, Hayashi M, Nishimura H, Tsurubuchi S (1989) Cross sections for collisions of electrons and photons with oxygen molecules. J Phys Chem Ref Data 18(1):23–42
Fu HB, Hu YJ, Bernstein ER (2006) Generation and detection of alkyl peroxy radicals in a supersonic jet expansion. J Chem Phys 125(1):014310
Meloni G, Zou P, Klippenstein SJ, Ahmed M, Leone SR, Taatjes CA, Osborn DL (2006) Energy-resolved photoionization of alkylperoxy radicals and the stability of their cations. J Am Chem Soc 128(41):13559–13567
Nixon KL, Pires WAD, Neves RFC, Duque HV, Jones DB, Brunger MJ, Lopes MCA (2016) Electron impact ionisation and fragmentation of methanol and ethanol. Int J Mass Spectrom 404:48–59
Zawadzki M (2018) Electron-impact ionization cross section of formic acid. Eur Phys J D 72(1):12
Vacher JR, Jorand F, Blin-Simiand N, Pasquiers S (2008) Partial ionization cross-sections of acetone and 2-butanone. Int J Mass Spectrom 273(3):117–125
Możejko P (2007) Calculations of electron impact ionization cross section for simple biomolecules: formic and acetic acids. Eur Phys J Spec Top 144(1):233–237
Große-Kreul S, Hübner S, Schneider S, Ellerweg D, von Keudell A, Matejčík S, Benedikt J (2015) Mass Spectrometry of Atmospheric Pressure Plasmas. Plasma Sources Sci Technol 24(4):044008
NIST (2010) Standard Reference Database 69: NIST Chemistry WebBook; National Institute of Standards and Technology
Loenders B, Engelmann Y, Bogaerts A (2021) Plasma-catalytic partial oxidation of methane on Pt(111): a microkinetic study on the role of different plasma species. J Phys Chem C 125(5):2966–2983
Eckert Z, Tsolas N, Togai K, Chernukho A, Yetter RA, Adamovich IV (2018) Kinetics of plasma-assisted oxidation of highly diluted hydrocarbon mixtures excited by a repetitive nanosecond pulse discharge. J Phys D Appl Phys 51(37):374002
Golda J, Biskup B, Layes V, Winzer T, Benedikt J (2020) Vacuum ultraviolet spectroscopy of cold atmospheric pressure plasma jets. Plasma Process Polym 17(6):1900216
Greenler RG (1962) Infrared study of the adsorption of methanol and ethanol on aluminum oxide. J Chem Phys 37(9):2094–2100
Korhonen ST, Bañares MA, Fierro JLG, Krause AOI (2007) Adsorption of methanol as a probe for surface characteristics of zirconia-, alumina-, and zirconia/alumina-supported chromia catalysts. Catal Today 126(1–2):235–247
Schauermann S, Hoffmann J, Johánek V, Hartmann J, Libuda J (2002) Adsorption, decomposition and oxidation of methanol on alumina supported palladium particles. Phys Chem Chem Phys 4(15):3909–3918
Plessis P, Marmet P, Dutil R (1983) Ionisation and appearance potentials of ch4 by electron impact. J Phys B At Mol Phys 16(7):1283–1294
Nozaki T, Muto N, Kado S, Okazaki K (2004) Dissociation of vibrationally excited methane on Ni catalyst: Part 1. Application to methane steam reforming. Catal Today 89:57–65
Nozière B, Hanson DR (2017) Speciated monitoring of gas-phase organic peroxy radicals by chemical ionization mass spectrometry: cross-reactions between CH3O2, CH3(CO)O2, (CH3)3CO2, and c-C6H11O2. J Phys Chem A 121(44):8453–8464
Jiang J, Kondeti SKVS, Nayak G, Bruggeman PJ (2021) Experimental and modeling studies of the plasma chemistry in a humid ar radiofrequency atmospheric pressure plasma jet. J. Phys. D. Appl. Phys. 55(22):225206
Agarwal N, Freakley SJ, McVicker RU, Althahban SM, Dimitratos N, He Q, Morgan DJ, Jenkins RL, Willock DJ, Taylor SH et al (2017) Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 358(6360):223–227
Benedikt J, Hecimovic A, Ellerweg D, von Keudell A (2012) Quadrupole mass spectrometry of reactive plasmas. J Phys D Appl Phys 45(40):403001
Acknowledgements
This material is based upon work supported by the National Science Foundation (CBET 1703439). The work has significantly benefited from methods and techniques developed in the framework of work supported by the US Department of Energy, Office of Science, Office of Fusion Energy Sciences General Plasma Science program under Award Number DE-SC0020232 and DE-SC0001939.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, J., Bruggeman, P.J. Investigation of the Mechanisms Underpinning Plasma-Catalyst Interaction for the Conversion of Methane to Oxygenates. Plasma Chem Plasma Process 42, 689–707 (2022). https://doi.org/10.1007/s11090-022-10251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-022-10251-5