Abstract
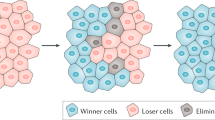
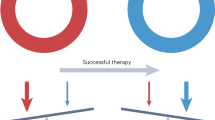
With the burgeoning use of immune-based treatments for cancer, never has there been a greater need to understand the tumour microenvironment within which immune cells function and how it can be perturbed to inhibit tumour growth. Yet, current challenges in identifying optimal combinations of immunotherapies and engineering new cell-based therapies highlight the limitations of conventional paradigms for the study of the tumour microenvironment. Ecology has a rich history of studying predator–prey dynamics to discern factors that drive prey to extinction. Here, we describe the basic tenets of predator–prey theory as applied to ‘predation’ by immune cells and the ‘extinction’ of cancer cells. Our synthesis reveals fundamental mechanisms by which antitumour immunity might fail in sometimes counterintuitive ways and provides a fresh yet evidence-based framework to better understand and therapeutically target the immune–cancer interface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nowell, P. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Hanna, N. H. et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 38, 1608–1632 (2020).
Seth, R. et al. Systemic therapy for melanoma: ASCO Guideline. J. Clin. Oncol. 38, 3947–3970 (2020).
Turtle, C. J. et al. CD19 CAR– T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123–2138 (2016).
Raje, N. et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380, 1726–1737 (2019).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19, 133–150 (2019).
Gatenby, R. A. A change of strategy in the war on cancer. Nature 459, 508–509 (2009).
Maley, C. C. et al. Classifying the evolutionary and ecological features of neoplasms. Nat. Rev. Cancer 17, 605–619 (2017).
Zhang, J., Cunningham, J. J., Brown, J. S. & Gatenby, R. A. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat. Commun. 8, 1816 (2017).
Gatenby, R. A., Zhang, J. & Brown, J. S. First strike–second strike strategies in metastatic cancer: lessons from the evolutionary dynamics of extinction. Cancer Res. 79, 3174–3177 (2019).
Kareva, I., Luddy, K. A., O’Farrelly, C., Gatenby, R. A. & Brown, J. S. Predator-prey in tumor-immune interactions: a wrong model or just an incomplete one? Front. Immunol. 12, 3391 (2021).
Cunningham, J. J., Brown, J. S., Gatenby, R. A. & Staňková, K. Optimal control to develop therapeutic strategies for metastatic castrate resistant prostate cancer. J. Theor. Biol. 459, 67–78 (2018).
Gatenby, R. A., Artzy-Randrup, Y., Epstein, T., Reed, D. R. & Brown, J. S. Eradicating metastatic cancer and the eco-evolutionary dynamics of Anthropocene extinctions. Cancer Res. 80, 613–623 (2020).
Holling, C. S. The components of predation as revealed by a study of small-mammal predation of the European Pine Sawfly1. Can. Entomol. 91, 293–320 (1959).
Kolumam, G. A., Thomas, S., Thompson, L. J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 (2004).
Jeschke, J. M. Density-dependent effects of prey defenses and predator offenses. J. Theor. Biol. 242, 900–907 (2006).
Jeschke, J. M., Kopp, M. & Tollrian, R. Predator functional responses: discriminating between handling and digesting prey. Ecol. Monogr. 72, 95–112 (2002).
Wiedemann, A., Depoil, D., Faroudi, M. & Valitutti, S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc. Natl Acad. Sci. USA 103, 10985–10990 (2006).
Bertrand, F. et al. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc. Natl Acad. Sci. USA 110, 6073–6078 (2013).
Khazen, R. et al. Functional heterogeneity of cytotoxic T cells and tumor resistance to cytotoxic hits limit anti-tumor activity in vivo. EMBO J. 40, e106658 (2021).
Breart, B., Lemaître, F., Celli, S. & Bousso, P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J. Clin. Invest. 118, 1390–1397 (2008).
Holling, C. S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398 (1959).
Eftimie, R., Bramson, J. L. & Earn, D. J. D. Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull. Math. Biol. 73, 2–32 (2011).
Jeschke, J. M., Kopp, M. & Tollrian, R. Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biol. Rev. 79, 337–349 (2004).
Kirschner, D. & Panetta, J. C. Modeling immunotherapy of the tumor - immune interaction. J. Math. Biol. 37, 235–252 (1998).
Johnston, C. J. C., Smyth, D. J., Dresser, D. W. & Maizels, R. M. TGF-β in tolerance, development and regulation of immunity. Cell. Immunol. 299, 14–22 (2015).
Kilgour, M. K. et al. 1-Methylnicotinamide is an immune regulatory metabolite in human ovarian cancer. Sci. Adv. 7, eabe1174 (2021).
Takahashi, F. Reproduction curve with two equilibrium points: a consideration on the fluctuation of insect population. Popul. Ecol. 6, 28–36 (1964).
Kuznetsov, V. A., Makalkin, I. A., Taylor, M. A. & Perelson, A. S. Nonlinear dynamics of immunogenic tumors: parameter estimation and global bifurcation analysis. Bull. Math. Biol. 56, 295–321 (1994).
Abrams, P. A. When does greater mortality increase population size? The long history and diverse mechanisms underlying the hydra effect. Ecol. Lett. 12, 462–474 (2009).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Huang, A. C. et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017).
Zappasodi, R., Merghoub, T. & Wolchok, J. D. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell 33, 581–598 (2018).
Joseph, R. W. et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin. Cancer Res. 24, 4960–4967 (2018).
Mueller, K. T. et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 130, 2317–2325 (2017).
Fultang, L. et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood 136, 1155–1160 (2020).
Polis, G. A., Myers, C. A. & Holt, R. D. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst. 20, 297–330 (1989).
Holt, R. D. & Polis, G. A. A theoretical framework for intraguild predation. Am. Nat. 149, 745–764 (1997).
Cressler, C. E., Nelson, W. A., Day, T. & McCauley, E. Disentangling the interaction among host resources, the immune system and pathogens. Ecol. Lett. 17, 284–293 (2014).
Kareva, I. & Berezovskaya, F. Cancer immunoediting: a process driven by metabolic competition as a predator–prey–shared resource type model. J. Theor. Biol. 380, 463–472 (2015).
Levin, S. A. The problem of pattern and scale in ecology: the Robert H. MacArthur Award Lecture. Ecology 73, 1943–1967 (1992).
Zhang, A. W. et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell 173, 1755–1769.e22 (2018).
Wijeyesinghe, S. et al. Expansible residence decentralizes immune homeostasis. Nature 592, 457–462 (2021).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019).
Poleszczuk, J. T. et al. Abscopal benefits of localized radiotherapy depend on activated T-cell trafficking and distribution between metastatic lesions. Cancer Res. 76, 1009–1018 (2016).
Ngwa, W. et al. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 18, 313–322 (2018).
Oliver, T. H. et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 30, 673–684 (2015).
Barrett, R. D. H. & Schluter, D. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (2008).
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, future. Cell 168, 613–628 (2017).
Miranda, A. et al. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl Acad. Sci. USA 116, 9020–9029 (2019).
Peplinski, J. et al. Ecology of fear: spines, armor and noxious chemicals deter predators in cancer and in nature. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2021.682504 (2021).
Hammill, E., Kratina, P., Vos, M., Petchey, O. L. & Anholt, B. R. Food web persistence is enhanced by non-trophic interactions. Oecologia 178, 549–556 (2015).
Kratina, P., Vos, M. & Anholt, B. R. Species diversity modulates predation. Ecology 88, 1917–1923 (2007).
Hillebrand, H. & Cardinale, B. J. Consumer effects decline with prey diversity. Ecol. Lett. 7, 192–201 (2004).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Rosenthal, R. et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485 (2019).
Wolf, Y. et al. UVB-induced tumor heterogeneity diminishes immune response in melanoma. Cell 179, 219–235.e21 (2019).
Denny, M. The fallacy of the average: on the ubiquity, utility and continuing novelty of Jensen’s inequality. J. Exp. Biol. 220, 139–146 (2017).
Okuyama, T. Individual behavioral variation in predator–prey models. Ecol. Res. 23, 665–671 (2008).
Yang, L., Pang, Y. & Moses, H. L. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 31, 220–227 (2010).
Webb, J. R., Milne, K., Kroeger, D. R. & Nelson, B. H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 141, 293–302 (2016).
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Marty Pyke, R. et al. Evolutionary pressure against MHC class II binding cancer mutations. Cell 175, 416–428.e13 (2018).
McGranahan, N. et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 171, 1259–1271.e11 (2017).
Van den Eynden, J., Jiménez-Sánchez, A., Miller, M. L. & Larsson, E. Lack of detectable neoantigen depletion signals in the untreated cancer genome. Nat. Genet. 51, 1741–1748 (2019).
Gatenby, R. A. & Brown, J. S. Integrating evolutionary dynamics into cancer therapy. Nat. Rev. Clin. Oncol. 17, 675–686 (2020).
Stearns, S. C., Ackermann, M., Doebeli, M. & Kaiser, M. Experimental evolution of aging, growth, and reproduction in fruitflies. Proc. Natl Acad. Sci. USA 97, 3309–3313 (2000).
Aktipis, C. A., Boddy, A. M., Gatenby, R. A., Brown, J. S. & Maley, C. C. Life history trade-offs in cancer evolution. Nat. Rev. Cancer 13, 883–892 (2013).
Giraldo, N. A. et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin. Cancer Res. 23, 4416–4428 (2017).
Hubbell, S. P. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 19, 166–172 (2005).
Sale, P. F. Coexistence of coral reef fishes — a lottery for living space. Environ. Biol. Fishes 3, 85–102 (1978).
Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 85, 183–206 (2010).
Sottoriva, A. et al. A big bang model of human colorectal tumor growth. Nat. Genet. 47, 209–216 (2015).
Williams, M. J., Werner, B., Barnes, C. P., Graham, T. A. & Sottoriva, A. Identification of neutral tumor evolution across cancer types. Nat. Genet. 48, 238–244 (2016).
Tarabichi, M. et al. Neutral tumor evolution? Nat. Genet. 50, 1630–1633 (2018).
Banchereau, R. et al. Molecular determinants of response to PD-L1 blockade across tumor types. Nat. Commun. 12, 3969 (2021).
Brook, B. W., Sodhi, N. S. & Bradshaw, C. J. A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (2008).
Lynch, M. & Gabriel, W. Mutation load and the survival of small populations. Evolution 44, 1725–1737 (1990).
Lynch, M., Conery, J. & Burger, R. Mutation accumulation and the extinction of small populations. Am. Nat. 146, 489–518 (1995).
Fagan, W. F. & Holmes, E. E. Quantifying the extinction vortex. Ecol. Lett. 9, 51–60 (2006).
Persi, E., Wolf, Y. I., Leiserson, M. D. M., Koonin, E. V. & Ruppin, E. criticality in tumor evolution and clinical outcome. Proc. Natl Acad. Sci. USA 115, E11101–E11110 (2018).
Persi, E. et al. Mutation-selection balance and compensatory mechanisms in tumour evolution. Nat. Rev. Genet. 22, 251–262 (2021).
McFarland, C. D., Korolev, K. S., Kryukov, G. V., Sunyaev, S. R. & Mirny, L. A. Impact of deleterious passenger mutations on cancer progression. Proc. Natl Acad. Sci. USA 110, 2910–2915 (2013).
McFarland, C. D., Mirny, L. A. & Korolev, K. S. Tug-of-war between driver and passenger mutations in cancer and other adaptive processes. Proc. Natl Acad. Sci. USA 111, 15138–15143 (2014).
Andor, N., Maley, C. C. & Ji, H. P. Genomic instability in cancer: teetering on the limit of tolerance. Cancer Res. 77, 2179–2185 (2017).
Talhouk, A. et al. Molecular subtype not immune response drives outcomes in endometrial carcinoma. Clin. Cancer Res. 25, 2537–2548 (2019).
Cescon, D. W., Bratman, S. V., Chan, S. M. & Siu, L. L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer 1, 276–290 (2020).
McArthur, H. L. et al. ALEXANDRA/IMpassion030: a phase III study of standard adjuvant chemotherapy with or without atezolizumab in early-stage triple-negative breast cancer. J. Clin. Oncol. 37, TPS598 (2019).
West, J. et al. The immune checkpoint kick start: optimization of neoadjuvant combination therapy using game theory. JCO Clin. Cancer Inform. 3, 1–12 (2019).
Park, D. S. et al. The goldilocks window of personalized chemotherapy: getting the immune response just right. Cancer Res. 79, 5302–5315 (2019).
Stinner, R. E. Efficacy of inundative releases. Annu. Rev. Entomol. 22, 515–531 (1977).
Cunningham, J. J. A call for integrated metastatic management. Nat. Ecol. Evol. 3, 996–998 (2019).
Hong, M., Clubb, J. D. & Chen, Y. Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 38, 473–488 (2020).
Anikeeva, N. et al. Efficient killing of tumor cells by CAR-T cells requires greater number of engaged CARs than TCRs. J. Biol. Chem. 297, 101033 (2021).
Hirayama, A. V. et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 133, 1876–1887 (2019).
Kochenderfer, J. N. et al. Lymphoma remissions caused by Anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 35, 1803–1813 (2017).
Hegde, M. et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 11, 3549 (2020).
Boulch, M. et al. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci. Immunol. 6, eabd4344 (2021).
Aspuria, P.-J. et al. An orthogonal IL-2 and IL-2Rβ system drives persistence and activation of CAR T cells and clearance of bulky lymphoma. Sci. Transl. Med. 13, eabg7565 (2021).
Zhang, Q. et al. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci. Transl. Med. 13, eabg6986 (2021).
MacKay, M. et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat. Biotechnol. 38, 233–244 (2020).
Schultz, L. M. et al. Phase 1 study of CD19/CD22 bispecific chimeric antigen receptor (CAR) therapy in children and young adults with B Cell acute lymphoblastic leukemia (ALL). Blood 132, 898 (2018).
Schneider, D. et al. Trispecific CD19-CD20-CD22–targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci. Transl. Med. 13, eabc6401 (2021).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Brunner-Weinzierl, M. C. & Rudd, C. E. CTLA-4 and PD-1 control of t-cell motility and migration: implications for tumor immunotherapy. Front. Immunol. 9, 2737 (2018).
Sharma, P. & Allison, J. P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 20, 75–76 (2020).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Oh, S. A. et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 1, 681–691 (2020).
Good-Jacobson, K. L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11, 535–542 (2010).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Vanhersecke, L. et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2, 794–802 (2021).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Robertson-Tessi, M., El-Kareh, A. & Goriely, A. A mathematical model of tumor–immune interactions. J. Theor. Biol. 294, 56–73 (2012).
Pagès, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003).
Kroeger, D. R., Milne, K. & Nelson, B. H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin. Cancer Res. 22, 3005–3015 (2016).
Wouters, M. C. A. & Nelson, B. H. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 24, 6125–6135 (2018).
Hu, X. et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat. Genet. 51, 560–567 (2019).
Roumenina, L. T., Daugan, M. V., Petitprez, F., Sautès-Fridman, C. & Fridman, W. H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019).
Lu, Y. et al. Complement signals determine opposite effects of B cell chemotherapy-induced immunity. Cell 180, 1081–1097.e24 (2020).
Turajlic, S. et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell 173, 595–610.e11 (2018).
AbdulJabbar, K. et al. Geospatial immune variability illuminates differential evolution of lung adenocarcinoma. Nat. Med. 26, 1054–1062 (2020).
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 173, 581–594.e12 (2018).
Tikhonov, G. et al. Joint species distribution modelling with the r-package Hmsc. Methods Ecol. Evol. 11, 442–447 (2020).
Warton, D. I. et al. So many variables: joint modeling in community ecology. Trends Ecol. Evol. 30, 766–779 (2015).
Lozupone, C. & Knight, R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235 (2005).
Niku, J., Hui, F. K. C., Taskinen, S. & Warton, D. I. gllvm: Fast analysis of multivariate abundance data with generalized linear latent variable models in R. Methods Ecol. Evol. 10, 2173–2182 (2019).
Legendre, P. & Legendre, L. Numerical Ecology (Elsevier, 2012).
Roberts, D. R. et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 40, 913–929 (2017).
Bolker, B. M. et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009).
Cross, W. et al. The evolutionary landscape of colorectal tumorigenesis. Nat. Ecol. Evol. 2, 1661–1672 (2018).
Nawaz, S., Heindl, A., Koelble, K. & Yuan, Y. Beyond immune density: critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod. Pathol. 28, 766–777 (2015).
Angelova, M. et al. Evolution of metastases in space and time under immune selection. Cell 175, 751–765.e16 (2018).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Acknowledgements
Earlier drafts of this work benefited greatly from input and discussion with P. Abrams, J. J. Lum, S. Perlman and A. M. Rodriguez. We apologize to the many authors whose work we were unable to cite owing to space constraints.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks J. Brown and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamilton, P.T., Anholt, B.R. & Nelson, B.H. Tumour immunotherapy: lessons from predator–prey theory. Nat Rev Immunol 22, 765–775 (2022). https://doi.org/10.1038/s41577-022-00719-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-022-00719-y
This article is cited by
-
Associations between HIFs and tumor immune checkpoints: mechanism and therapy
Discover Oncology (2024)
-
BEST: a web application for comprehensive biomarker exploration on large-scale data in solid tumors
Journal of Big Data (2023)
-
Modeling the Role of Immune Cell Conversion in the Tumor-Immune Microenvironment
Bulletin of Mathematical Biology (2023)