Abstract
Purpose
To assess the direction and strength of climate and leaf litter trait effects on decomposition dynamics throughout the litter decomposition process.
Methods
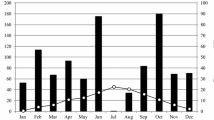
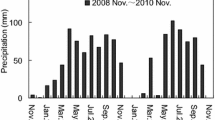
We performed a three-year-long litterbag translocation experiment along an elevational gradient in a transitional mixed forest in China. We explored temporal shifts in the relative contribution of microclimate and litter traits of seven dominant species to mass loss throughout the decomposition process.
Results
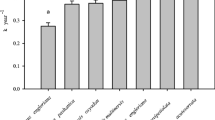
Air temperature and soil moisture imparted no significant effects on mass loss in the initial decomposition stage (0–6 months) but exerted positive effects after 6 months’ incubation (p < 0.05). Initial specific leaf area (SLA) was positively associated with mass loss only during the early decomposition stages (0–12 months). Litter P concentration was positively while N concentration was negatively associated with mass loss for almost all decomposition stages. Both litter Mg and Ca concentrations were negatively associated with mass loss throughout the whole decomposition process (p < 0.05). The relative contribution of microclimate was weaker than that of litter traits during the early stages but increased in the late decomposition stage (after 36 months). SLA and Mg were the most important traits during the early stages (0-12 months), whereas N and Mg were relatively stronger during the later stages (30–36 months).
Conclusion
Our results have highlighted that microclimate (particularly temperature) exerted dominant control over later-stage litter decomposition. Often-overlooked litter traits such as Mg, are key potential drivers of litter decomposition dynamics and should be more explicitly incorporated into current biogeochemical models to better understand litter-driven nutrient and carbon cycling.





Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
The code analyzed during the current study are available from the corresponding author upon reasonable request.
References
Adair EC, Hobbie SE, Hobbie RK (2010) Single-pool exponential decomposition models: potential pitfalls in their use in ecological studies. Ecology 91:1225–1236
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Averill C, Waring B (2018) Nitrogen limitation of decomposition and decay: How can it occur? Global Change Biol 24:1417–1427. https://doi.org/10.1111/gcb.13980
Bartoń K (2020). MuMIn: Multi-Model Inference. R package version 1.43.17. Available via https://CRAN.R-project.org/package=MuMIn
Berg B (2014) Decomposition patterns for foliar litter–A theory for influencing factors. Soil Biol Biochem 78:222–232
Berg B, Kjønaas OJ, Johansson MB, Erhagen B, Åkerblom S (2015) Late stage pine litter decomposition: Relationship to litter N, Mn, and acid unhydrolyzable residue (AUR) concentrations and climatic factors. Forest Ecol Manag 358:41–47
Berg B, McClaugherty C (2020) Plant litter-decomposition, humus formation and carbon sequestration. Springer Nature, Switzerland
Berg B, Sun T, Johansson M-B, Sanborn P, Ni X, Åkerblom S, Lönn M (2021) Magnesium dynamics in decomposing foliar litter-A synthesis. Geoderma 382:114756. https://doi.org/10.1016/j.geoderma.2020.114756
Bradford MA, Berg B, Maynard DS, Wieder WR, Wood SA (2016) Understanding the dominant controls on litter decomposition. J Ecol 104:229–238. https://doi.org/10.1111/1365-2745.12507
Bradford MA, Veen GF, Bonis A, Bradford EM, Classen AT, Cornelissen JHC, Crowther TW, De Long JR, Freschet GT, Kardol P, Manrubia-Freixa M, Maynard DS, Newman GS, Logtestijn RSP, Viketoft M, Wardle DA, Wieder WR, Wood SA, van der Putten WH (2017) A test of the hierarchical model of litter decomposition. Nature Ecology & Evolution 1:1836–1845. https://doi.org/10.1038/s41559-017-0367-4
Bradford MA, Warren Ii RJ, Baldrian P, Crowther TW, Maynard DS, Oldfield EE, Wieder WR, Wood SA, King JR (2014) Climate fails to predict wood decomposition at regional scales. Nat Clim Change 4:625–630. https://doi.org/10.1038/nclimate2251
Bütikofer L, Anderson K, Bebber DP, Bennie JJ, Early RI, Maclean IMD (2020) The problem of scale in predicting biological responses to climate. Global Change Biol 26:6657–6666. https://doi.org/10.1111/gcb.15358
Canessa R, van den Brink L, Saldaña A, Rios RS, Hättenschwiler S, Mueller CW, Prater I, Tielbörger K, Bader MY (2021) Relative effects of climate and litter traits on decomposition change with time, climate and trait variability. J Ecol 109:447–458. https://doi.org/10.1111/1365-2745.13516
Chomel M, Guittonny-Larchevêque M, Fernandez C, Gallet C, DesRochers A, Paré D, Jackson BG, Baldy V (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104:1527–1541. https://doi.org/10.1111/1365-2745.12644
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. https://doi.org/10.1111/j.1461-0248.2008.01219.x
Cornwell WK, Weedon JT (2014) Decomposition trajectories of diverse litter types: a model selection analysis. Methods Ecol Evol 5:173–182
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779. https://doi.org/10.1038/ngeo2520
Coûteaux M-M, Bottner P, Berg B (1995) Litter decomposition, climate and liter quality. Trends Ecol Evol 10:63–66. https://doi.org/10.1016/S0169-5347(00)88978-8
Currie W, Harmon M, Burke I, Hart S, Parton W, Silver W (2010) Cross-biome transplants of plant litter show decomposition models extend to a broader climatic range but lose predictability at the decadal time scale. Global Change Biol 16:1744–1761
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Global Change Biol 15:1339–1355
Cusack DF, Karpman J, Ashdown D, Cao Q, Ciochina M, Halterman S, Lydon S, Neupane A (2016) Global change effects on humid tropical forests: Evidence for biogeochemical and biodiversity shifts at an ecosystem scale. Rev Geophys 54:523–610
Dale S, Turner B, Bardgett R (2015) Isolating the effects of precipitation, soil conditions, and litter quality on leaf litter decomposition in lowland tropical forests. Plant Soil 394:225–238. https://doi.org/10.1007/s11104-015-2511-8
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Fox J, Weisberg S (2019) An R Companion to Applied Regression, Third Edition. Sage: Thousand Oaks. URL: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Fujii S, Cornelissen JH, Berg MP, Mori AS (2018) Tree leaf and root traits mediate soil faunal contribution to litter decomposition across an elevational gradient. Funct Ecol 32:840–852
García-Palacios P, Gross N, Gaitán J, Maestre FT (2018) Climate mediates the biodiversity–ecosystem stability relationship globally. Proc Natl Acad Sci 115:8400–8405. https://doi.org/10.1073/pnas.1800425115
García-Palacios P, Maestre FT, Kattge J, Wall DH (2013) Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett 16:1045–1053. https://doi.org/10.1111/ele.12137
García-Palacios P, Shaw EA, Wall DH, Hättenschwiler S (2016) Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecol Lett 19:554–563
Ge J, Berg B, Xie Z (2019) Climatic seasonality is linked to the occurrence of the mixed evergreen and deciduous broad-leaved forests in China. Ecosphere 10:e02862. https://doi.org/10.1002/ecs2.2862
Ge J, Wang Y, Xu W, Xie Z (2017) Latitudinal patterns and climatic drivers of leaf litter multiple nutrients in Chinese broad-leaved tree species: Does leaf habit matter? Ecosystems 20:1124–1136. https://doi.org/10.1007/s10021-016-0098-4
Ge J, Xie Z (2017) Geographical and climatic gradients of evergreen versus deciduous broadleaved tree species in subtropical China: Implications for the definition of the mixed forest. Ecol Evol 7:3636–3644. https://doi.org/10.1002/ece3.2967
Ge J, Xiong G, Zhao C, Shen G, Xie Z (2013) Short-term dynamic shifts in woody plants in a montane mixed evergreen and deciduous broadleaved forest in central China. Forest Ecol Manag 310:740–746. https://doi.org/10.1016/j.foreco.2013.09.019
Ge J, Xu W, Xiong G, Zhao C, Li J, Liu Q, Tang Z, Xie Z (2022) Depth-dependent controls over soil organic carbon stock across Chinese shrublands. Ecosystems. https://doi.org/10.1007/s10021-022-00757-6
Gilliam FS (2016) Forest ecosystems of temperate climatic regions: from ancient use to climate change. New Phytol 212:871–887. https://doi.org/10.1111/nph.14255
Goebel M, Hobbie SE, Bulaj B, Zadworny M, Archibald DD, Oleksyn J, Reich PB, Eissenstat DM (2011) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monogr 81:89–102
Gross N, Bagousse-Pinguet YL, Liancourt P, Berdugo M, Gotelli NJ, Maestre FT (2017) Functional trait diversity maximizes ecosystem multifunctionality. Nature Ecology & Evolution 1:0132. https://doi.org/10.1038/s41559-017-0132
Hättenschwiler S, Coq S, Barantal S, Handa IT (2011) Leaf traits and decomposition in tropical rainforests: revisiting some commonly held views and towards a new hypothesis. New Phytol 189:950–965. https://doi.org/10.1111/j.1469-8137.2010.03483.x
Hättenschwiler S, Jørgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763. https://doi.org/10.1111/j.1365-2745.2010.01671.x
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30:357–363. https://doi.org/10.1016/j.tree.2015.03.015
Hoeber S, Fransson P, Weih M, Manzoni S (2020) Leaf litter quality coupled to Salix variety drives litter decomposition more than stand diversity or climate. Plant Soil 453:313–328. https://doi.org/10.1007/s11104-020-04606-0
Jiang L, Wang H, Li S, Fu X, Dai X, Yan H, Kou L (2021) Mycorrhizal and environmental controls over root trait–decomposition linkage of woody trees. New Phytol 229:284–295. https://doi.org/10.1111/nph.16844
Keiser AD, Bradford MA (2017) Climate masks decomposer influence in a cross-site litter decomposition study. Soil Biol Biochem 107:180–187. https://doi.org/10.1016/j.soilbio.2016.12.022
Keller AB, Phillips RP (2019) Leaf litter decay rates differ between mycorrhizal groups in temperate, but not tropical, forests. New Phytol 222:556–564
Kou L, Jiang L, Hättenschwiler S, Zhang M, Niu S, Fu X, Dai X, Yan H, Li S, Wang H (2020) Diversity-Decomposition Relationships in Forests Worldwide Elife 9:e55813. https://doi.org/10.7554/eLife.55813
Li Q, Zhang M, Geng Q, Jin C, Zhu J, Ruan H, Xu X (2020) The roles of initial litter traits in regulating litter decomposition: a “common plot” experiment in a subtropical evergreen broadleaf forest. Plant Soil 452:207–216. https://doi.org/10.1007/s11104-020-04563-8
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041. https://doi.org/10.1111/j.1461-0248.2012.01826.x
Manzoni S, Piñeiro G, Jackson RB, Jobbágy EG, Kim JH, Porporato A (2012) Analytical models of soil and litter decomposition: solutions for mass loss and time-dependent decay rates. Soil Biol Biochem 50:66–76
Marklein AR, Winbourne JB, Enders SK, Gonzalez DJX, van Huysen TL, Izquierdo JE, Light DR, Liptzin D, Miller KE, Morford SL, Norton RA, Houlton BZ (2016) Mineralization ratios of nitrogen and phosphorus from decomposing litter in temperate versus tropical forests. Global Ecol Biogeogr 25:335–346. https://doi.org/10.1111/geb.12414
Moore TR, Trofymow JA, Prescott CE, Titus BD (2017) Can short-term litter-bag measurements predict long-term decomposition in northern forests? Plant Soil 416:419–426. https://doi.org/10.1007/s11104-017-3228-7
Oberle B, Lee MR, Myers JA, Osazuwa-Peters OL, Spasojevic MJ, Walton ML, Young DF, Zanne AE (2020) Accurate forest projections require long-term wood decay experiments because plant trait effects change through time. Global Change Biol 26:864–875. https://doi.org/10.1111/gcb.14873
Parsons SA, Congdon RA, Lawler IR (2014) Determinants of the pathways of litter chemical decomposition in a tropical region. New Phytol 203:873–882. https://doi.org/10.1111/nph.12852
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234. https://doi.org/10.1071/BT12225
Powers JS, Salute S (2011) Macro-and micronutrient effects on decomposition of leaf litter from two tropical tree species: inferences from a short-term laboratory incubation. Plant Soil 346:245–257
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available via http://www.R-project.org/
Rowley MC, Grand S, Verrecchia ÉP (2018) Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 137:27–49
Salinas N, Malhi Y, Meir P, Silman M, Roman Cuesta R, Huaman J, Salinas D, Huaman V, Gibaja A, Mamani M (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189:967–977
Santiago LS (2007) Extending the leaf economics spectrum to decomposition: evidence from a tropical forest. Ecology 88:1126–1131
Santonja M, Milcu A, Fromin N, Rancon A, Shihan A, Fernandez C, Baldy V, Hättenschwiler S (2019) Temporal shifts in plant diversity effects on carbon and nitrogen dynamics during litter decomposition in a Mediterranean shrubland exposed to reduced precipitation. Ecosystems 22:939–954. https://doi.org/10.1007/s10021-018-0315-4
See CR, Luke McCormack M, Hobbie SE, Flores-Moreno H, Silver WL, Kennedy PG (2019) Global patterns in fine root decomposition: climate, chemistry, mycorrhizal association and woodiness. Ecol Lett 22:946–953. https://doi.org/10.1111/ele.13248
Shen G, Chen D, Wu Y, Liu L, Liu C (2019) Spatial patterns and estimates of global forest litterfall. Ecosphere 10:e02587. https://doi.org/10.1002/ecs2.2587
Sun T, Hobbie SE, Berg B, Zhang H, Wang Q, Wang Z, Hättenschwiler S (2018) Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc Natl Acad Sci 115:10392–10397. https://doi.org/10.1073/pnas.1716595115
Sundqvist MK, Sanders NJ, Wardle DA (2013) Community and ecosystem responses to evational gradients: processes, mechanisms, and insights for global change. Annu Rev Ecol Evol Syst 44:261–280
Suseela V, Tharayil N (2018) Decoupling the direct and indirect effects of climate on plant litter decomposition: Accounting for stress-induced modifications in plant chemistry. Global Change Biol 24:1428–1451. https://doi.org/10.1111/gcb.13923
Suseela V, Tharayil N, Xing B, Dukes JS (2013) Labile compounds in plant litter reduce the sensitivity of decomposition to warming and altered precipitation. New Phytol 200:122–133
The Editorial Board of Flora of China (2004) Flora of China. Science Press, Beijing
Waring BG (2012) A meta-analysis of climatic and chemical controls on leaf litter decay rates in tropical forests. Ecosystems 15:999–1009
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90:3333–3341
Wiesmeier M, Urbanski L, Hobley E, Lang B, von Lützow M, Marin-Spiotta E, van Wesemael B, Rabot E, Ließ M, Garcia-Franco N (2019) Soil organic carbon storage as a key function of soils-A review of drivers and indicators at various scales. Geoderma 333:149–162
Wu Z (1980) Vegetation of China. Science Press, Beijing
Xie Z, Shen G (2021) The outstanding universal value and conservation of Hubei Shennongjia: The World Natural Heritage Site. Springer Nature.
Zanne AE, Oberle B, Dunham KM, Milo AM, Walton ML, Young DF (2015) A deteriorating state of affairs: How endogenous and exogenous factors determine plant decay rates. J Ecol 103:1421–1431
Zhou S, Butenschoen O, Barantal S, Handa IT, Makkonen M, Vos V, Aerts R, Berg MP, McKie B, Van Ruijven J, Hättenschwiler S, Scheu S (2020) Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J Ecol 108:2283–2297. https://doi.org/10.1111/1365-2745.13452
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Science & Business Media.
Acknowledgements
We wish to acknowledge all participants for their contributions to the fieldwork and laboratory analysis in this study. We would like to thank Professor Björn Berg at the University of Helsinki for his insightful comments on this manuscript. We also would like to thank Dr. Morgan Furze at Yale University and Dr. Shannon Elliot at Michigan State University for their assistance with the English language and grammatical editing. The National Key Research and Development Program of China (Grant No. 2019YFD1100403), The National Natural Science Foundation of China (Grant No.31600360), and The State Key Laboratory of Vegetation and Environmental Change of China (Grant No. Y7206F1016) financed this study.
Funding
Both the National Key Research and Development Program of China (Grant No. 2019YFD1100403) and the National Natural Science Foundation of China (Grant No.31600360) financed this study.
Author information
Authors and Affiliations
Contributions
J.G. and Z.X. conceived of and designed study, performed research, and wrote the manuscript. J.G., B.M., B.B., W.X., and C.Z analyzed data and revised this manuscript. All the authors approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Alfonso Escudero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ge, J., Ma, B., Xu, W. et al. Temporal shifts in the relative importance of climate and leaf litter traits in driving litter decomposition dynamics in a Chinese transitional mixed forest. Plant Soil 477, 679–692 (2022). https://doi.org/10.1007/s11104-022-05425-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05425-1