Abstract
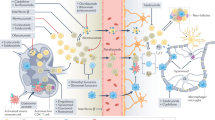
Our incomplete understanding of the causes and pathways involved in the onset and progression of multiple sclerosis (MS) limits our ability to effectively treat this complex neurological disease. Recent studies explore the role of immune cells at different stages of MS and how they interact with cells of the central nervous system (CNS). The findings presented here begin to question the exclusivity of an antigen-specific cause and highlight how seemingly distinct immune cell types can share common functions that drive disease. Innovative techniques further expose new disease-associated immune cell populations and reinforce how environmental context is critical to their phenotype and subsequent role in disease. Importantly, the differentiation of immune cells into a pathogenic state is potentially reversible through therapeutic manipulation. As such, understanding the mechanisms that provide plasticity to causal cell types is likely key to uncoupling these disease processes and may identify novel therapeutic targets that replace the need for cell ablation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, eaav7188 (2019). This work presents fine-mapped GWAS data that reveal how the immune system, in particular T cells, NK cells and microglia, are the primary drivers in MS genetic susceptibility.
Alfredsson, L. & Olsson, T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb. Perspect. Med. 9, a028944 (2019).
Jacobs, B. M. et al. Gene–environment interactions in multiple sclerosis: a UK Biobank study. Neurol. Neuroimmunol. Neuroinflamm. 8, e1007 (2021).
Ramien, C. et al. Sex effects on inflammatory and neurodegenerative processes in multiple sclerosis. Neurosci. Biobehav. Rev. 67, 137–146 (2016).
Khalil, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14, 577–589 (2018).
Bjornevik, K. et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 77, 58–64 (2019).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein–Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022). This high-powered study is the first to confirm that EBV infection is directly associated with, and precedes, the development of MS.
Carassiti, D. et al. Neuronal loss, demyelination and volume change in the multiple sclerosis neocortex. Neuropathol. Appl. Neurobiol. 44, 377–390 (2018).
Scalfari, A. et al. The cortical damage, early relapses, and onset of the progressive phase in multiple sclerosis. Neurology 90, e2107–e2118 (2018).
Reay, W. R. & Cairns, M. J. Advancing the use of genome-wide association studies for drug repurposing. Nat. Rev. Genet. 22, 658–671 (2021).
King, E. A., Davis, J. W. & Degner, J. F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 15, e1008489 (2019).
Gregory, A. P. et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488, 508–511 (2012). This study reveals why anti-TNF therapy for MS causes disease worsening, in contrast to the benefits observed in treating other autoimmune diseases.
Moutsianas, L. et al. Class II HLA interactions modulate genetic risk for multiple sclerosis. Nat. Genet. 47, 1107–1113 (2015).
van Oosten, B. W. et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology 49, 351–357 (1997).
Mundt, S. et al. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 4, eaau8380 (2019).
Kaur, G., Trowsdale, J. & Fugger, L. Natural killer cells and their receptors in multiple sclerosis. Brain 136, 2657–2676 (2013).
International Multiple Sclerosis Genetics Consortium. A systems biology approach uncovers cell-specific gene regulatory effects of genetic associations in multiple sclerosis. Nat. Commun. 10, 2236 (2019).
Gresle, M. M. et al. Multiple sclerosis risk variants regulate gene expression in innate and adaptive immune cells. Life Sci. Alliance 3, e202000650 (2020).
Dendrou, C. A. et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci. Transl. Med. 8, 363ra149 (2016). This study demonstrates how functional genomics can be used to understand biological processes that can inform drug development pipelines.
Benton, M. L. et al. The influence of evolutionary history on human health and disease. Nat. Rev. Genet. 22, 269–283 (2021).
Lünemann, J. D. et al. Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann. Neurol. 67, 159–169 (2010).
Pender, M. P., Csurhes, P. A., Burrows, J. M. & Burrows, S. R. Defective T-cell control of Epstein–Barr virus infection in multiple sclerosis. Clin. Transl. Immunol. 6, e126 (2017).
Engdahl, E. et al. Increased serological response against human herpesvirus 6A is associated with risk for multiple sclerosis. Front. Immunol. 10, 2715 (2019).
Handel, A. E. et al. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS ONE 5, e12496 (2010).
Endriz, J., Ho, P. P. & Steinman, L. Time correlation between mononucleosis and initial symptoms of MS. Neurol. Neuroimmunol. Neuroinflamm 4, e308 (2017).
Jacobs, B. M., Giovannoni, G., Cuzick, J. & Dobson, R. Systematic review and meta-analysis of the association between Epstein–Barr virus, multiple sclerosis and other risk factors. Mult. Scler. 26, 1281–1297 (2020).
Ochoa-Repáraz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009).
Rizzo, F. et al. Interferon-β therapy specifically reduces pathogenic memory B cells in multiple sclerosis patients by inducing a FAS-mediated apoptosis. Immunol. Cell Biol. 94, 886–894 (2016).
Aksoy, S. et al. Rituximab-related viral infections in lymphoma patients. Leuk. Lymphoma 48, 1307–1312 (2007).
Perlejewski, K. et al. Search for viral agents in cerebrospinal fluid in patients with multiple sclerosis using real-time PCR and metagenomics. PLoS ONE 15, e0240601 (2020).
Cermelli, C. et al. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J. Infect. Dis. 187, 1377–1387 (2003).
Veroni, C., Serafini, B., Rosicarelli, B., Fagnani, C. & Aloisi, F. Transcriptional profile and Epstein–Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J. Neuroinflammation 15, 18 (2018).
Serafini, B. et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204, 2899–2912 (2007).
Hedström, A. K., Olsson, T. & Alfredsson, L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult. Scler. 18, 1334–1336 (2012).
Wesnes, K. et al. Body size and the risk of multiple sclerosis in Norway and Italy: the EnvIMS study. Mult. Scler. 21, 388–395 (2015).
Bosch-Queralt, M. et al. Diet-dependent regulation of TGFβ impairs reparative innate immune responses after demyelination. Nat. Metab. 3, 211–227 (2021).
Berer, K. et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl Acad. Sci. USA 114, 10719–10724 (2017).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Colpitts, S. L. et al. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 8, 561–573 (2017).
Duscha, A. et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 180, 1067–1080.e16 (2020).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018).
Jensen, S. N. et al. Isoflavone diet ameliorates experimental autoimmune encephalomyelitis through modulation of gut bacteria depleted in patients with multiple sclerosis. Sci. Adv. 7, eabd4595 (2021).
Cignarella, F. et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 27, 1222–1235.e6 (2018).
Zhang, D. et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity 51, 671–681.e5 (2019).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Na, S.-Y., Janakiraman, M., Leliavski, A. & Krishnamoorthy, G. High-salt diet suppresses autoimmune demyelination by regulating the blood–brain barrier permeability. Proc. Natl Acad. Sci. USA 118, e2025944118 (2021).
Matthias, J. et al. Salt generates antiinflammatory TH17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. J. Clin. Invest. 130, 4587–4600 (2020).
Parks, N. E., Jackson-Tarlton, C. S., Vacchi, L., Merdad, R. & Johnston, B. C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 5, CD004192 (2020).
Brown, J. W. L. et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 321, 175–187 (2019).
He, A. et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 19, 307–316 (2020).
Kunkl, M., Frascolla, S., Amormino, C., Volpe, E. & Tuosto, L. T helper cells: the modulators of inflammation in multiple sclerosis. Cells 9, 482 (2020).
Schafflick, D. et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat. Commun. 11, 247 (2020).
Tuzlak, S. et al. Repositioning TH cell polarization from single cytokines to complex help. Nat. Immunol. 22, 1210–1217 (2021).
Murphy, A. C., Lalor, S. J., Lynch, M. A. & Mills, K. H. G. Infiltration of TH1 and TH17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 24, 641–651 (2010).
Tahmasebinia, F. & Pourgholaminejad, A. The role of TH17 cells in auto-inflammatory neurological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 79, 408–416 (2017).
Wekerle, H., Kojima, K., lannes-Vieira, J., Lassmann, H. & Linington, C. Animal models. Ann. Neurol. 36, S47–S53 (1994).
Cruciani, C. et al. T-cell specificity influences disease heterogeneity in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm 8, e1075 (2021).
Lodygin, D. et al. β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature 566, 503–508 (2019). This study provides evidence to support a role for immune cells in grey matter pathology, indicating how the immune system may cause neuronal decline and contribute directly to neurodegeneration.
Kaufmann, M. et al. Identifying CNS-colonizing T cells as potential therapeutic targets to prevent progression of multiple sclerosis. Med 2, 296–312.e8 (2021). This study harnesses the ability of natalizumab to trap pathogenic immune cells in the periphery to reveal their identity and phenotype.
Galli, E. et al. GM-CSF and CXCR4 define a T helper cell signature in multiple sclerosis. Nat. Med. 25, 1290–1300 (2019).
Cano-Gamez, E. et al. Single-cell transcriptomics identifies an effectorness gradient shaping the response of CD4+ T cells to cytokines. Nat. Commun. 11, 1801 (2020).
Kiner, E. et al. Gut CD4+ T cell phenotypes are a continuum molded by microbes, not by TH archetypes. Nat. Immunol. 22, 216–228 (2021).
Hiltensperger, M. et al. Skin and gut imprinted helper T cell subsets exhibit distinct functional phenotypes in central nervous system autoimmunity. Nat. Immunol. 22, 880–892 (2021).
Yu, N. et al. CD4+CD25+CD127low/– T cells: a more specific Treg population in human peripheral blood. Inflammation 35, 1773–1780 (2012).
Kohm, A. P., Carpentier, P. A., Anger, H. A. & Miller, S. D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169, 4712–4716 (2002).
Venken, K. et al. Compromised CD4+CD25high regulatory T-cell function in patients with relapsing–remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 123, 79–89 (2008).
Dominguez-Villar, M., Baecher-Allan, C. M. & Hafler, D. A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 17, 673–675 (2011).
Sumida, T. et al. Activated β-catenin in Foxp3+ regulatory T cells links inflammatory environments to autoimmunity. Nat. Immunol. 19, 1391–1402 (2018).
Lucca, L. E. et al. TIGIT signaling restores suppressor function of TH1 Tregs. JCI Insight 4, e124427 (2019).
Verma, N. D. et al. Multiple sclerosis patients have reduced resting and increased activated CD4+CD25+FOXP3+ T regulatory cells. Sci. Rep. 11, 10476 (2021).
Dominguez-Villar, M., Walker, L. S. K. & Piconese, S. Control of Regulatory T Cell Stability, Plasticity and Function in Health and Disease (Frontiers Media SA, 2021).
Zhao, H., Liao, X. & Kang, Y. Tregs: where we are and what comes next? Front. Immunol. 8, 1578 (2017).
Mascanfroni, I. D. et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 21, 638–646 (2015).
Astier, A. L. & Hafler, D. A. Abnormal Tr1 differentiation in multiple sclerosis. J. Neuroimmunol. 191, 70–78 (2007).
Dankers, W. et al. Human memory TH17 cell populations change into anti-inflammatory cells with regulatory capacity upon exposure to active vitamin D. Front. Immunol. 10, 1504 (2019).
Farez, M. F. et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162, 1338–1352 (2015).
Mitsdoerffer, M. & Kuchroo, V. New pieces in the puzzle: how does interferon-β really work in multiple sclerosis? Ann. Neurol. 65, 487–488 (2009).
Babbe, H. et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192, 393–404 (2000).
Machado-Santos, J. et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141, 2066–2082 (2018).
Fransen, N. L. et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 143, 1714–1730 (2020).
Pender, M. P., Csurhes, P. A., Pfluger, C. M. & Burrows, S. R. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult. Scler. 20, 1825–1832 (2014).
Smolders, J. et al. Tissue-resident memory T cells populate the human brain. Nat. Commun. 9, 4593 (2018).
Tzartos, J. S. et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172, 146–155 (2008).
Huseby, E. S. et al. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J. Exp. Med. 194, 669–676 (2001).
van Nierop, G. P. et al. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 134, 383–401 (2017).
Lanz, T. V. et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603, 321–327 (2022). This work presents direct evidence showing autoimmune cross-reactivity between an EBV protein and a CNS-specific protein as a potential cause for MS.
Sabolek, M. K. et al. Communication of CD8+ T cells with mononuclear phagocytes in multiple sclerosis. Ann. Clin. Transl. Neurol. 6, 1151–1164 (2019).
Ji, Q., Castelli, L. & Goverman, J. M. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8+ T cells. Nat. Immunol. 14, 254–261 (2013).
Page, N. et al. Persistence of self-reactive CD8+ T cells in the CNS requires TOX-dependent chromatin remodeling. Nat. Commun. 12, 1009 (2021).
Najafian, N. et al. Regulatory functions of CD8+CD28– T cells in an autoimmune disease model. J. Clin. Invest. 112, 1037–1048 (2003).
Jiang, H., Zhang, S. I. & Pernis, B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science 256, 1213–1215 (1992).
Baughman, E. J. et al. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J. Autoimmun. 36, 115–124 (2011).
Cunnusamy, K. et al. Disease exacerbation of multiple sclerosis is characterized by loss of terminally differentiated autoregulatory CD8+ T cells. Clin. Immunol. 152, 115–126 (2014).
Pannemans, K. et al. HLA-E restricted CD8+ T cell subsets are phenotypically altered in multiple sclerosis patients. Mult. Scler. 20, 790–801 (2014).
Karandikar, N. J. et al. Glatiramer acetate (Copaxone) therapy induces CD8+ T cell responses in patients with multiple sclerosis. J. Clin. Invest. 109, 641–649 (2002).
Tennakoon, D. K. et al. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J. Immunol. 176, 7119–7129 (2006).
Saligrama, N. et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572, 481–487 (2019). This work describes the expansion of regulatory CD8+ T cells generated in a coordinated effort to counteract autoreactive CD4+ T cells and γδ T cells in EAE, a phenomenon that is predicted to occur in patients with MS and has also been observed in coeliac disease.
Florou, D., Katsara, M., Feehan, J., Dardiotis, E. & Apostolopoulos, V. Anti-CD20 agents for multiple sclerosis: spotlight on ocrelizumab and ofatumumab. Brain Sci. 10, 758 (2020).
Cencioni, M. T., Mattoscio, M., Magliozzi, R., Bar-Or, A. & Muraro, P. A. B cells in multiple sclerosis — from targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 17, 399–414 (2021).
Serafini, B., Rosicarelli, B., Magliozzi, R., Stigliano, E. & Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–174 (2004).
Kuenz, B. et al. Cerebrospinal fluid B cells correlate with early brain inflammation in multiple sclerosis. PLoS ONE 3, e2559 (2008).
Villar, L. M. et al. Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Ann. Neurol. 76, 231–240 (2014).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007).
von Büdingen, H.-C. et al. B cell exchange across the blood–brain barrier in multiple sclerosis. J. Clin. Invest. 122, 4533–4543 (2012).
Palanichamy, A. et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci. Transl. Med. 6, 248ra106 (2014).
Stern, J. N. H. et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci. Transl. Med. 6, 248ra107 (2014).
Brändle, S. M. et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc. Natl Acad. Sci. USA 113, 7864–7869 (2016).
Mancuso, R. et al. Effects of natalizumab on oligoclonal bands in the cerebrospinal fluid of multiple sclerosis patients: a longitudinal study. Mult. Scler. 20, 1900–1903 (2014).
Wang, J. et al. HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 183, 1264–1281.e20 (2020). This work shows that memory B cells from patients who are HLA-DRB1*15:01+ can activate CD4+ T cells in the absence of exogenous peptides by presenting self-peptides, including RASGRP2, and peptides derived from HLA-DRB1*15:01 and HLA-DRB5*01:01 molecules themselves.
Lisak, R. P. et al. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. J. Neuroimmunol. 246, 85–95 (2012).
Lisak, R. P. et al. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J. Neuroimmunol. 309, 88–99 (2017).
Brioschi, S. et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 373, eabf9277 (2021).
Rojas, O. L. et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 176, 610–624.e18 (2019).
Mitsdoerffer, M. et al. Formation and immunomodulatory function of meningeal B cell aggregates in progressive CNS autoimmunity. Brain 144, 1697–1710 (2021).
McKenzie, D. R. et al. IL-17-producing γδ T cells switch migratory patterns between resting and activated states. Nat. Commun. 8, 15632 (2017).
Ammitzbøll, C. et al. MAIT cell subtypes in multiple sclerosis. J. Neuroimmunol. 339, 577117 (2020).
De Biasi, S. et al. iNKT cells in secondary progressive multiple sclerosis patients display pro-inflammatory profiles. Front. Immunol. 7, 555 (2016).
Rinaldi, L. et al. Longitudinal analysis of immune cell phenotypes in early stage multiple sclerosis: distinctive patterns characterize MRI-active patients. Brain 129, 1993–2007 (2006).
Hvas, J., Oksenberg, J. R., Fernando, R., Steinman, L. & Bernard, C. C. γδ T cell receptor repertoire in brain lesions of patients with multiple sclerosis. J. Neuroimmunol. 46, 225–234 (1993).
Stinissen, P. et al. Increased frequency of γδ T cells in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. Reactivity, cytotoxicity, and T cell receptor V gene rearrangements. J. Immunol. 154, 4883–4894 (1995). This study provides evidence to support a role for unconventional T cells in MS pathogenesis.
Shimonkevitz, R., Colburn, C., Burnham, J. A., Murray, R. S. & Kotzin, B. L. Clonal expansions of activated γδ T cells in recent-onset multiple sclerosis. Proc. Natl Acad. Sci. USA 90, 923–927 (1993).
Freedman, M. S., Buu, N. N., Ruijs, T. C., Williams, K. & Antel, J. P. Differential expression of heat shock proteins by human glial cells. J. Neuroimmunol. 41, 231–238 (1992).
Zeine, R. et al. Mechanism of γδ T cell-induced human oligodendrocyte cytotoxicity: relevance to multiple sclerosis. J. Neuroimmunol. 87, 49–61 (1998).
Schirmer, L., Rothhammer, V., Hemmer, B. & Korn, T. Enriched CD161highCCR6+ γδ T cells in the cerebrospinal fluid of patients with multiple sclerosis. JAMA Neurol. 70, 345–351 (2013).
Singh, A. K. et al. High interferon-γ uniquely in Vδ1 T cells correlates with markers of inflammation and axonal damage in early multiple sclerosis. Front. Immunol. 8, 260 (2017).
Willing, A., Jäger, J., Reinhardt, S., Kursawe, N. & Friese, M. A. Production of IL-17 by MAIT cells is increased in multiple sclerosis and is associated with IL-7 receptor expression. J. Immunol. 200, 974–982 (2018).
Illés, Z., Shimamura, M., Newcombe, J., Oka, N. & Yamamura, T. Accumulation of Vα7.2-Jα33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int. Immunol. 16, 223–230 (2004).
Abrahamsson, S. V. et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 136, 2888–2903 (2013).
Held, K. et al. αβ T-cell receptors from multiple sclerosis brain lesions show MAIT cell-related features. Neurol. Neuroimmunol. Neuroinflamm 2, e107 (2015).
Willing, A. et al. CD8+ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur. J. Immunol. 44, 3119–3128 (2014).
Salou, M. et al. Neuropathologic, phenotypic and functional analyses of mucosal associated invariant T cells in multiple sclerosis. Clin. Immunol. 166–167, 1–11 (2016).
Carnero Contentti, E., Farez, M. F. & Correale, J. Mucosal-associated invariant T cell features and TCR repertoire characteristics during the course of multiple sclerosis. Front. Immunol. 10, 2690 (2019).
Ammitzbøll, C. et al. Smoking reduces circulating CD26hiCD161hi MAIT cells in healthy individuals and patients with multiple sclerosis. J. Leukoc. Biol. 101, 1211–1220 (2017).
Mexhitaj, I. et al. Abnormal effector and regulatory T cell subsets in paediatric-onset multiple sclerosis. Brain 142, 617–632 (2019).
Dias, J., Leeansyah, E. & Sandberg, J. K. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl Acad. Sci. USA 114, E5434–E5443 (2017).
Illés, Z. et al. Differential expression of NK T cell Vα24JαQ invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 164, 4375–4381 (2000).
O’Keeffe, J. et al. T-cells expressing natural killer (NK) receptors are altered in multiple sclerosis and responses to α-galactosylceramide are impaired. J. Neurol. Sci. 275, 22–28 (2008).
Gigli, G., Caielli, S., Cutuli, D. & Falcone, M. Innate immunity modulates autoimmunity: type 1 interferon-beta treatment in multiple sclerosis promotes growth and function of regulatory invariant natural killer T cells through dendritic cell maturation. Immunol. 122, 409–417 (2007).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Kwong, B. et al. T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat. Immunol. 18, 1117–1127 (2017).
Eken, A. et al. Fingolimod alters tissue distribution and cytokine production of human and murine innate lymphoid cells. Front. Immunol. 10, 217 (2019).
Grigg, J. B. et al. Antigen-presenting innate lymphoid cells orchestrate neuroinflammation. Nature 600, 707–712 (2021). This work shows that antigen presentation by inflammatory ILC3s is required to promote T cell responses in the CNS and the development of MS-like disease in mouse models.
Russi, A. E., Ebel, M. E., Yang, Y. & Brown, M. A. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc. Natl Acad. Sci. USA 115, E1520–E1529 (2018).
Hirose, S. et al. Type 2 innate lymphoid cells induce CNS demyelination in an HSV-IL-2 mouse model of multiple sclerosis. iScience 23, 101549 (2020).
Gross, C. C. et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc. Natl Acad. Sci. USA 113, E2973–E2982 (2016).
Han, S. et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J. Immunol. 192, 2551–2563 (2014).
Rodríguez-Martín, E. et al. Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin. Exp. Immunol. 180, 243–249 (2015).
Caruana, P., Lemmert, K., Ribbons, K., Lea, R. & Lechner-Scott, J. Natural killer cell subpopulations are associated with MRI activity in a relapsing–remitting multiple sclerosis patient cohort from Australia. Mult. Scler. 23, 1479–1487 (2017).
Saraste, M., Irjala, H. & Airas, L. Expansion of CD56bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-β. Neurol. Sci. 28, 121–126 (2007).
Martínez-Rodríguez, J. E. et al. Natural killer cell phenotype and clinical response to interferon-β therapy in multiple sclerosis. Clin. Immunol. 141, 348–356 (2011).
Gross, C. C. et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm 3, e289 (2016).
Medina, S. et al. Optimal response to dimethyl fumarate associates in MS with a shift from an inflammatory to a tolerogenic blood cell profile. Mult. Scler. 24, 1317–1327 (2018).
Montes Diaz, G., Fraussen, J., Van Wijmeersch, B., Hupperts, R. & Somers, V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci. Rep. 8, 8194 (2018).
Skarica, M., Eckstein, C., Whartenby, K. A. & Calabresi, P. A. Novel mechanisms of immune modulation of natalizumab in multiple sclerosis patients. J. Neuroimmunol. 235, 70–76 (2011).
Darlington, P. J. et al. Natural killer cells regulate TH17 cells after autologous hematopoietic stem cell transplantation for relapsing remitting multiple sclerosis. Front. Immunol. 9, 834 (2018).
Nielsen, N., Ødum, N., Ursø, B., Lanier, L. L. & Spee, P. Cytotoxicity of CD56bright NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS ONE 7, e31959 (2012).
Morandi, F. et al. Intrathecal soluble HLA-E correlates with disease activity in patients with multiple sclerosis and may cooperate with soluble HLA-G in the resolution of neuroinflammation. J. Neuroimmune Pharmacol. 8, 944–955 (2013).
Plantone, D. et al. Circulating CD56dim NK cells expressing perforin are increased in progressive multiple sclerosis. J. Neuroimmunol. 265, 124–127 (2013).
Mishra, M. K., Wang, J., Silva, C., Mack, M. & Yong, V. W. Kinetics of proinflammatory monocytes in a model of multiple sclerosis and its perturbation by laquinimod. Am. J. Pathol. 181, 642–651 (2012).
Cugurra, A. et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 373, eabf7844 (2021). This work presents evidence in mice supporting that a large proportion of continuously replenished myeloid cells in the dura mater are not blood derived but, rather, transit from cranial bone marrow through specialized channels.
Akaishi, T., Takahashi, T. & Nakashima, I. Peripheral blood monocyte count at onset may affect the prognosis in multiple sclerosis. J. Neuroimmunol. 319, 37–40 (2018).
Kouwenhoven, M., Teleshova, N., Ozenci, V., Press, R. & Link, H. Monocytes in multiple sclerosis: phenotype and cytokine profile. J. Neuroimmunol. 112, 197–205 (2001).
Makhlouf, K., Weiner, H. L. & Khoury, S. J. Increased percentage of IL-12+ monocytes in the blood correlates with the presence of active MRI lesions in MS. J. Neuroimmunol. 119, 145–149 (2001).
Fiedler, S. E., Spain, R. I., Kim, E. & Salinthone, S. Lipoic acid modulates inflammatory responses of monocytes and monocyte-derived macrophages from healthy and relapsing–remitting multiple sclerosis patients. Immunol. Cell Biol. 99, 107–115 (2021).
Spain, R. et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol. Neuroimmunol. Neuroinflamm 4, e374 (2017).
Guilliams, M., Mildner, A. & Yona, S. Developmental and functional heterogeneity of monocytes. Immunity 49, 595–613 (2018).
Locatelli, G. et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat. Neurosci. 21, 1196–1208 (2018).
Giladi, A. et al. Cxcl10+ monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol. 21, 525–534 (2020). This study identifies two pathogenic monocyte subsets expressing CXCL10 and SAA3, which may be candidates for targeted therapeutic intervention.
Blecher-Gonen, R. et al. Single-cell analysis of diverse pathogen responses defines a molecular roadmap for generating antigen-specific immunity. Cell Syst. 8, 109–121.e6 (2019).
White, M. P. J., Webster, G., Leonard, F. & La Flamme, A. C. Innate IFN-γ ameliorates experimental autoimmune encephalomyelitis and promotes myeloid expansion and PDL-1 expression. Sci. Rep. 8, 259 (2018).
Smith, B. C., Sinyuk, M., Jenkins, J. E., Psenicka, M. W. & Williams, J. L. The impact of regional astrocyte interferon-γ signaling during chronic autoimmunity: a novel role for the immunoproteasome. J. Neuroinflammation 17, 184 (2020).
King, I. L., Dickendesher, T. L. & Segal, B. M. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 113, 3190–3197 (2009).
Lotfi, N., Zhang, G.-X., Esmaeil, N. & Rostami, A. Evaluation of the effect of GM-CSF blocking on the phenotype and function of human monocytes. Sci. Rep. 10, 1567 (2020).
Lee, K. M. C., Achuthan, A. A. & Hamilton, J. A. GM-CSF: a promising target in inflammation and autoimmunity. Immunotargets Ther. 9, 225–240 (2020).
Constantinescu, C. S. et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2, e117 (2015).
Haas, J., Schwarz, A., Korporal-Kuhnke, M., Jarius, S. & Wildemann, B. Myeloid dendritic cells exhibit defects in activation and function in patients with multiple sclerosis. J. Neuroimmunol. 301, 53–60 (2016).
Thewissen, K. et al. Circulating dendritic cells of multiple sclerosis patients are proinflammatory and their frequency is correlated with MS-associated genetic risk factors. Mult. Scler. 20, 548–557 (2014).
Krumbholz, M. et al. CCL19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J. Neuroimmunol. 190, 72–79 (2007).
Barcellos, L. F. et al. CC-chemokine receptor 5 polymorphism and age of onset in familial multiple sclerosis. Multiple Sclerosis Genetics Group. Immunogenetics 51, 281–288 (2000).
Gade-Andavolu, R. et al. Association of CCR5 Δ32 deletion with early death in multiple sclerosis. Genet. Med. 6, 126–131 (2004).
Karni, A. et al. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 177, 4196–4202 (2006).
Ko, H.-J. et al. GM-CSF-responsive monocyte-derived dendritic cells are pivotal in TH17 pathogenesis. J. Immunol. 192, 2202–2209 (2014).
Nutt, S. L. & Chopin, M. Transcriptional networks driving dendritic cell differentiation and function. Immunity 52, 942–956 (2020).
Sie, C. & Korn, T. Dendritic cells in central nervous system autoimmunity. Semin. Immunopathol. 39, 99–111 (2017).
Segal, B. M. et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing–remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7, 796–804 (2008).
Mondal, S. et al. IL-12 p40 monomer is different from other IL-12 family members to selectively inhibit IL-12Rβ1 internalization and suppress EAE. Proc. Natl Acad. Sci. USA 117, 21557–21567 (2020).
Schwab, N., Zozulya, A. L., Kieseier, B. C., Toyka, K. V. & Wiendl, H. An imbalance of two functionally and phenotypically different subsets of plasmacytoid dendritic cells characterizes the dysfunctional immune regulation in multiple sclerosis. J. Immunol. 184, 5368–5374 (2010).
Balashov, K. E., Aung, L. L., Vaknin-Dembinsky, A., Dhib-Jalbut, S. & Weiner, H. L. Interferon-β inhibits Toll-like receptor 9 processing in multiple sclerosis. Ann. Neurol. 68, 899–906 (2010).
Stasiolek, M. et al. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain 129, 1293–1305 (2006).
Huang, Y. et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21, 530–540 (2018).
Krogsgaard, M. et al. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)–DR2–MBP 85–99 complex. J. Exp. Med. 191, 1395–1412 (2000).
Wolf, Y. et al. Microglial MHC class II is dispensable for experimental autoimmune encephalomyelitis and cuprizone-induced demyelination. Eur. J. Immunol. 48, 1308–1318 (2018).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Tozaki-Saitoh, H. et al. Transcription factor MafB contributes to the activation of spinal microglia underlying neuropathic pain development. Glia 67, 729–740 (2019).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021). This work uses barcoded viral tracing with single-cell RNA sequencing to identify novel mediators of microglia–astrocyte interactions that promote CNS pathology in EAE and, potentially, MS.
Wheeler, M. A. et al. MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599 (2020).
Sanmarco, L. M., Polonio, C. M., Wheeler, M. A. & Quintana, F. J. Functional immune cell–astrocyte interactions. J. Exp. Med. 218, e20202715 (2021).
Medawar, P. B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 29, 58–69 (1948).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Absinta, M. et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 6, e29738 (2017).
Giles, D. A., Duncker, P. C., Wilkinson, N. M., Washnock-Schmid, J. M. & Segal, B. M. CNS-resident classical DCs play a critical role in CNS autoimmune disease. J. Clin. Invest. 128, 5322–5334 (2018).
Sage, P. T. et al. Dendritic cell PD-L1 limits autoimmunity and follicular T cell differentiation and function. J. Immunol. 200, 2592–2602 (2018).
Filippi, M. et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain 142, 1858–1875 (2019).
Woo, M. S. et al. Neuronal metabotropic glutamate receptor 8 protects against neurodegeneration in CNS inflammation. J. Exp. Med. 218, e20201290 (2021).
International Multiple Sclerosis Genetics Consortium. Genome-wide association study of severity in multiple sclerosis. Genes. Immun. 12, 615–625 (2011).
Absinta, M. et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 76, 1474–1483 (2019).
Elliott, C. et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 142, 2787–2799 (2019).
Bevan, R. J., Evans, R., Griffiths, L. & Watkins, L. M. Meningeal inflammation and cortical demyelination in acute multiple sclerosis. Ann. Neurol. 84, 829–842 (2018).
Choi, S. R., Howell, O. W., Carassiti, D. & Magliozzi, R. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–2937 (2012).
Schläger, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Pitzalis, C., Jones, G. W., Bombardieri, M. & Jones, S. A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14, 447–462 (2014).
Zhan, J., Kipp, M., Han, W. & Kaddatz, H. Ectopic lymphoid follicles in progressive multiple sclerosis: from patients to animal models. Immunology 164, 450–466 (2021).
Magliozzi, R., Howell, O. W. & Reeves, C. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 68, 477–493 (2010).
Schirmer, L. et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82 (2019). This work uses single-nucleus RNA-sequencing of MS brain tissue to identify lineage and region-specific transcriptomic changes associated with selective cortical neuron damage and glial activation contributing to progression of MS lesions.
Wagner, C. A., Roqué, P. J. & Goverman, J. M. Pathogenic T cell cytokines in multiple sclerosis. J. Exp. Med. 217, e20190460 (2020).
Yates, R. L. et al. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann. Neurol. 82, 259–270 (2017).
Ryu, J. K. et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat. Immunol. 19, 1212–1223 (2018).
Werneburg, S. et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52, 167–182.e7 (2020).
Mahad, D., Ziabreva, I., Lassmann, H. & Turnbull, D. Mitochondrial defects in acute multiple sclerosis lesions. Brain 131, 1722–1735 (2008).
Friese, M. A., Schattling, B. & Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 10, 225–238 (2014).
Kaufmann, T. et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 22, 1617–1623 (2019).
Eshaghi, A. et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain 141, 1665–1677 (2018).
Peng, C., Trojanowski, J. Q. & Lee, V. M.-Y. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 16, 199–212 (2020).
Schattling, B. et al. Bassoon proteinopathy drives neurodegeneration in multiple sclerosis. Nat. Neurosci. 22, 887–896 (2019).
Ontaneda, D., Thompson, A. J., Fox, R. J. & Cohen, J. A. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet 389, 1357–1366 (2017).
Wang, A., Rojas, O., Lee, D. & Gommerman, J. L. Regulation of neuroinflammation by B cells and plasma cells. Immunol. Rev. 299, 45–60 (2021).
Quinn, J. L., Kumar, G., Agasing, A., Ko, R. M. & Axtell, R. C. Role of TFH cells in promoting T helper 17-induced neuroinflammation. Front. Immunol. 9, 382 (2018).
Guo, J. et al. T follicular helper-like cells are involved in the pathogenesis of experimental autoimmune encephalomyelitis. Front. Immunol. 9, 944 (2018).
Plastini, M. J., Desu, H. L. & Brambilla, R. Dynamic responses of microglia in animal models of multiple sclerosis. Front. Cell. Neurosci. 14, 269 (2020).
Malik, S., Want, M. Y. & Awasthi, A. The emerging roles of γδ T cells in tissue inflammation in experimental autoimmune encephalomyelitis. Front. Immunol. 7, 14 (2016).
Croxford, J. L., Miyake, S., Huang, Y.-Y., Shimamura, M. & Yamamura, T. Invariant Vα19i T cells regulate autoimmune inflammation. Nat. Immunol. 7, 987–994 (2006).
Cui, Y. & Wan, Q. NKT cells in neurological diseases. Front. Cell. Neurosci. 13, 245 (2019).
Steinbach, K. et al. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J. Exp. Med. 213, 1571–1587 (2016).
Urban, S. L. et al. Peripherally induced brain tissue-resident memory CD8+ T cells mediate protection against CNS infection. Nat. Immunol. 21, 938–949 (2020). This work shows that peripheral infections generate antigen-specific CD8+ memory T cells in the brain that adopt a unique tissue-resident memory signature.
Lassmann, H. Multiple sclerosis pathology. Cold Spring Harb. Perspect. Med. 8, a028936 (2018).
Prineas, J. W. & Parratt, J. D. E. Oligodendrocytes and the early multiple sclerosis lesion. Ann. Neurol. 72, 18–31 (2012).
Kuhlmann, T. et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017).
Spelman, T. et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing–remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 78, 1197–1204 (2021).
Spath, S. et al. Dysregulation of the cytokine GM-CSF induces spontaneous phagocyte invasion and immunopathology in the central nervous system. Immunity 46, 245–260 (2017).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Seyedmirzaei, H. & Rezaei, N. Cytokine alterations in psoriasis: an updated review. Expert Rev. Clin. Immunol. 17, 1323–1335 (2021).
Papp, K. et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N. Engl. J. Med. 379, 1313–1321 (2018).
Afrasiabi, A. et al. The interaction of human and Epstein–Barr virus miRNAs with multiple sclerosis risk loci. Int. J. Mol. Sci. 22, 2927 (2021).
Ricigliano, V. A. G. et al. EBNA2 binds to genomic intervals associated with multiple sclerosis and overlaps with vitamin D receptor occupancy. PLoS ONE 10, e0119605 (2015). This study shows that direct interaction of environmental factors with regions of the genome that contain MS-associated SNPs supports the significance of the gene–environment axis in disease cause.
Afrasiabi, A., Parnell, G. P., Swaminathan, S., Stewart, G. J. & Booth, D. R. The interaction of multiple sclerosis risk loci with Epstein–Barr virus phenotypes implicates the virus in pathogenesis. Sci. Rep. 10, 193 (2020).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to discussion of the content. K.E.A., L.T.J. and M.A.F. wrote the article. M.K. designed the figures. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks B. Becher, T. Korn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Relapsing–remitting MS
-
(RRMS). Fluctuations in multiple sclerosis (MS) disease activity involving periods of complete or partial symptom relief (remission) between clinical episodes (relapse).
- Primary progressive MS
-
(PPMS). Clinical worsening of multiple sclerosis (MS) disease from onset, without periods of clinical improvement (remissions).
- Natalizumab
-
Monoclonal antibody against the integrin very late antigen 4 (VLA4) that is used to treat patients with relapsing–remitting multiple sclerosis (RRMS).
- Grey matter
-
Brain tissue that largely consists of unmyelinated, neuronal cell bodies.
- White matter
-
Brain tissue that largely consists of myelinated neuronal axons.
- Secondary progressive MS
-
(SPMS). Patients with relapsing–remitting multiple sclerosis (RRMS) will typically progress into a secondary progressive phase, in which clinical worsening persists without any periods of remission.
- T follicular helper cells
-
(TFH cells). A specialist subset of CD4+ T cells that promote the generation of germinal centres within secondary lymphoid organs where they support B cell proliferation and their development into antibody-producing plasma cells.
- Glatiramer acetate
-
An immunomodulatory drug used to treat relapsing–remitting multiple sclerosis (RMMS), consisting of synthetic polypeptides composed of four amino acids resembling myelin basic protein (MBP).
- Unconventional T cells
-
T cells characterized by their ability to raise public responses to a wide range of antigens compared with the highly specific T cell receptor (TCR) repertoire expressed by conventional T cells.
- NKT cells
-
A subset of CD1d-restricted T cells that express both T cell receptors (TCRs) and receptors of the natural killer (NK) cell lineage.
- Innate lymphoid cells
-
(ILCs). Cells that differentiate from a common innate lymphoid progenitor through the expression of specific transcription factors, with most populations (excluding natural killer (NK) cells), subsequently residing within tissues where they contribute to immune defence and tissue homeostasis.
- Fingolimod
-
A sphingosine-1-phosphate receptor (S1PR) modulator that prevents the movement of lymphocytes out of lymph nodes; used as a treatment for patients with relapsing–remitting multiple sclerosis (RMMS).
- Clinically isolated syndrome
-
(CIS). A clinical event in which neurological symptoms, involving inflammation and/or demyelination in the central nervous system (CNS), last for a period greater than 24 h, which then either fully or partially resolves. CIS may precede the onset of other neurological diseases, including multiple sclerosis (MS).
Rights and permissions
About this article
Cite this article
Attfield, K.E., Jensen, L.T., Kaufmann, M. et al. The immunology of multiple sclerosis. Nat Rev Immunol 22, 734–750 (2022). https://doi.org/10.1038/s41577-022-00718-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-022-00718-z
This article is cited by
-
Neutrophil–lymphocyte ratio and platelet–lymphocyte ratio as predictors of MS severity: a retrospective cohort study
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2024)
-
A repair pathway lost in multiple sclerosis provides a new drug opportunity
Nature Immunology (2024)
-
The immunopathogenesis of narcolepsy type 1
Nature Reviews Immunology (2024)
-
Ancient DNA reveals evolutionary origins of autoimmune diseases
Nature Reviews Immunology (2024)
-
Childhood and adolescence factors and multiple sclerosis: results from the German National Cohort (NAKO)
BMC Neurology (2024)