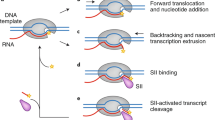
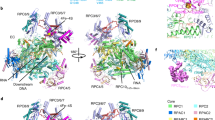
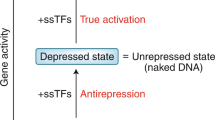
Abstract
The eukaryotic transcription apparatus synthesizes a staggering diversity of RNA molecules. The labour of nuclear gene transcription is, therefore, divided among multiple DNA-dependent RNA polymerases. RNA polymerase I (Pol I) transcribes ribosomal RNA, Pol II synthesizes messenger RNAs and various non-coding RNAs (including long non-coding RNAs, microRNAs and small nuclear RNAs) and Pol III produces transfer RNAs and other short RNA molecules. Pol I, Pol II and Pol III are large, multisubunit protein complexes that associate with a multitude of additional factors to synthesize transcripts that largely differ in size, structure and abundance. The three transcription machineries share common characteristics, but differ widely in various aspects, such as numbers of RNA polymerase subunits, regulatory elements and accessory factors, which allows them to specialize in transcribing their specific RNAs. Common to the three RNA polymerases is that the transcription process consists of three major steps: transcription initiation, transcript elongation and transcription termination. In this Review, we outline the common principles and differences between the Pol I, Pol II and Pol III transcription machineries and discuss key structural and functional insights obtained into the three stages of their transcription processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Roeder, R. G. & Rutter, W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature 224, 234–237 (1969).
Roeder, R. G. & Rutter, W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc. Natl Acad. Sci. USA 65, 675–682 (1970).
Abraham, K. J. et al. Nucleolar RNA polymerase II drives ribosome biogenesis. Nature 585, 298–302 (2020).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III–transcribed RNA intermediate. Nat. Immunol. 10, 1065–1072 (2009).
Chiu, Y.-H., MacMillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Palazzo, A. F. & Lee, E. S. Non-coding RNA: what is functional and what is junk? Front. Genet. 6, 2 (2015).
Zhou, M. & Law, J. A. RNA Pol IV and V in gene silencing: rebel polymerases evolving away from Pol II’s rules. Curr. Opin. Plant. Biol. 27, 154–164 (2015).
Cramer, P., Bushnell, D. A. & Kornberg, R. D. Structural basis of transcription: RNA polymerase II at 2.8 ångstrom resolution. Science 292, 1863–1876 (2001).
Gnatt, A. L., Cramer, P., Fu, J., Bushnell, D. A. & Kornberg, R. D. Structural basis of transcription: an RNA polymerase II elongation complex at 3.3Å resolution. Science 292, 1876–1882 (2001).
Fernández-Tornero, C. et al. Crystal structure of the 14-subunit RNA polymerase I. Nature 502, 644–649 (2013).
Engel, C., Sainsbury, S., Cheung, A. C., Kostrewa, D. & Cramer, P. RNA polymerase I structure and transcription regulation. Nature 502, 650–655 (2013).
Hoffmann, N. A. et al. Molecular structures of unbound and transcribing RNA polymerase III. Nature 528, 231–236 (2015).
Misiaszek, A. D. et al. Cryo-EM structures of human RNA polymerase I. Nat. Struct. Mol. Biol. 28, 997–1008 (2021).
Zhao, D. et al. Structure of the human RNA polymerase I elongation complex. Cell Discov. 7, 97 (2021).
Bernecky, C., Herzog, F., Baumeister, W., Plitzko, J. M. & Cramer, P. Structure of transcribing mammalian RNA polymerase II. Nature 529, 551–554 (2016).
Girbig, M. et al. Cryo-EM structures of human RNA polymerase III in its unbound and transcribing states. Nat. Struct. Mol. Biol. 28, 210–219 (2021).
Ramsay, E. P. et al. Structure of human RNA polymerase III. Nat. Commun. 11, 6409 (2020).
Wang, Q. et al. Structural insights into transcriptional regulation of human RNA polymerase III. Nat. Struct. Mol. Biol. 28, 220–227 (2021).
Li, L. et al. Structure of human RNA polymerase III elongation complex. Cell Res. 31, 791–800 (2021).
Edwards, A. M., Kane, C. M., Young, R. A. & Kornberg, R. D. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J. Biol. Chem. 266, 71–75 (1991).
Jasiak, A. J., Armache, K. J., Martens, B., Jansen, R. P. & Cramer, P. Structural biology of RNA polymerase III: subcomplex C17/25 X-ray structure and 11 subunit enzyme model. Mol. Cell 23, 71–81 (2006).
Allison, L. A., Moyle, M., Shales, M. & James Ingles, C. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell 42, 599–610 (1985).
McCracken, S. et al. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385, 357–361 (1997).
Kuhn, C.-D. D. et al. Functional architecture of RNA polymerase I. Cell 131, 1260–1272 (2007).
Geiger, S. R. et al. RNA polymerase I contains a TFIIF-related DNA-binding subcomplex. Mol. Cell 39, 583–594 (2010).
Vorländer, M. K., Khatter, H., Wetzel, R., Hagen, W. J. H. & Müller, C. W. Molecular mechanism of promoter opening by RNA polymerase III. Nature 553, 295–300 (2018).
Abascal-Palacios, G., Ramsay, E. P., Beuron, F., Morris, E. & Vannini, A. Structural basis of RNA polymerase III transcription initiation. Nature 553, 301–306 (2018).
Han, Y., Yan, C., Fishbain, S., Ivanov, I. & He, Y. Structural visualization of RNA polymerase III transcription machineries. Cell Discov. 4, 40 (2018).
Chédin, S., Riva, M., Schultz, P., Sentenac, A. & Carles, C. The RNA cleavage activity of RNA polymerase III is mediated by an essential TFIIS-like subunit and is important for transcription termination. Genes Dev. 12, 3857–3871 (1998).
Kornberg, R. D. Chromatin structure: a repeating unit of histones and DNA. Science 184, 868–871 (1974).
Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 (1997).
Andersson, R. & Sandelin, A. Determinants of enhancer and promoter activities of regulatory elements. Nat. Rev. Genet. 21, 71–87 (2020).
Birch, J. L. & Zomerdijk, J. C. B. M. Structure and function of ribosomal RNA gene chromatin. Biochem. Soc. Trans. 36, 619–624 (2008).
Németh, A. & Längst, G. Genome organization in and around the nucleolus. Trends Genet. 27, 149–156 (2011).
Goodfellow, S. J. & Zomerdijk, J. C. B. M. Basic mechanisms in RNA polymerase I transcription of the ribosomal RNA genes. Subcell. Bochem. 61, 211–236 (2012).
McStay, B. & Grummt, I. The epigenetics of rRNA genes: from molecular to chromosome biology. Annu. Rev. Cell Dev. Biol. 24, 131–157 (2008).
Radebaugh, C. A., Gong, X., Bartholomew, B. & Paule, M. R. Identification of previously unrecognized common elements in eukaryotic promoters: a ribosomal RNA gene initiator element for RNA polymerase I. J. Biol. Chem. 272, 3141–3144 (1997).
Panov, K. I., Friedrich, J. K., Russell, J. & Zomerdijk, J. C. B. M. UBF activates RNA polymerase I transcription by stimulating promoter escape. EMBO J. 25, 3310–3322 (2006).
Knutson, B. A., Smith, M. L., Belkevich, A. E. & Fakhouri, A. M. Molecular topology of RNA polymerase I upstream activation factor. Mol. Cell. Biol. 40, e00056–20 (2020).
Grummt, I. Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev. 17, 1691–1702 (2003).
Gonzalez, I. L. & Sylvester, J. E. Complete sequence of the 43-kb human ribosomal DNA repeat: analysis of the intergenic spacer. Genomics 27, 320–328 (1995).
Grimaldi, G. & Di Nocera, P. P. Multiple repeated units in Drosophila melanogaster ribosomal DNA spacer stimulate rRNA precursor transcription. Proc. Natl Acad. Sci. USA 85, 5502–5506 (1988).
Mayer, C., Schmitz, K.-M., Li, J., Grummt, I. & Santoro, R. Intergenic transcripts regulate the epigenetic state of rRNA genes. Mol. Cell 22, 351–361 (2006).
Santoro, R., Schmitz, K. M., Sandoval, J. & Grummt, I. Intergenic transcripts originating from a subclass of ribosomal DNA repeats silence ribosomal RNA genes in trans. EMBO Rep. 11, 52–58 (2010).
Németh, A., Guibert, S., Tiwari, V. K., Ohlsson, R. & Längst, G. Epigenetic regulation of TTF-I-mediated promoter–terminator interactions of rRNA genes. EMBO J. 27, 1255–1265 (2008).
Sylvester, J. E. et al. The human ribosomal RNA genes: structure and organization of the complete repeating unit. Hum. Genet. 73, 193–198 (1986).
Parks, M. M. et al. Variant ribosomal RNA alleles are conserved and exhibit tissue-specific expression. Sci. Adv. 4, eaao0665 (2018).
Gagnon-Kugler, T., Langlois, F., Stefanovsky, V., Lessard, F. & Moss, T. Loss of human ribosomal gene CpG methylation enhances cryptic RNA polymerase II transcription and disrupts ribosomal RNA processing. Mol. Cell 35, 414–425 (2009).
Zentner, G. E., Saiakhova, A., Manaenkov, P., Adams, M. D. & Scacheri, P. C. Integrative genomic analysis of human ribosomal DNA. Nucleic Acids Res. 39, 4949–4960 (2011).
Maiser, A. et al. Super-resolution in situ analysis of active ribosomal DNA chromatin organization in the nucleolus. Sci. Rep. 10, 1–11 (2020).
Ngoc, L. V., Cassidy, C. J., Huang, C. Y., Duttke, S. H. C. & Kadonaga, J. T. The human initiator is a distinct and abundant element that is precisely positioned in focused core promoters. Genes Dev. 31, 6–11 (2017).
Ngoc, L. V., Kassavetis, G. A. & Kadonaga, J. T. The RNA polymerase II core promoter in Drosophila. Genetics 212, 13–24 (2019).
Haberle, V. & Stark, A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 19, 621–637 (2018).
Yang, C., Bolotin, E., Jiang, T., Sladek, F. M. & Martinez, E. Prevalence of the initiator over the TATA box in human and yeast genes and identification of DNA motifs enriched in human TATA-less core promoters. Gene 389, 52–65 (2007).
Deng, W. & Roberts, S. G. E. A core promoter element downstream of the TATA box that is recognized by TFIIB. Genes Dev. 19, 2418–2423 (2005).
Kutach, A. K. & Kadonaga, J. T. The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol. Cell. Biol. 20, 4754–4764 (2000).
Sloutskin, A., Shir-Shapira, H., Freiman, R. N. & Juven-Gershon, T. The core promoter is a regulatory hub for developmental gene expression. Front. Cell Dev. Biol. 9, 2557 (2021).
Guiro, J. & Murphy, S. Regulation of expression of human RNA polymerase II-transcribed snRNA genes. Open Biol. 7, 170073 (2017).
Lim, C. Y. et al. The MTE, a new core promoter element for transcription by RNA polymerase II. Genes Dev. 18, 1606–1617 (2004).
Lee, D.-H. et al. Functional characterization of core promoter elements: the downstream core element is recognized by TAF1. Mol. Cell. Biol. 25, 9674–9686 (2005).
Tokusumi, Y., Ma, Y., Song, X., Jacobson, R. H. & Takada, S. The new core promoter element XCPE1 (X core promoter element 1) directs activator-, mediator-, and TATA-binding protein-dependent but TFIID-independent RNA polymerase II transcription from TATA-less promoters. Mol. Cell. Biol. 27, 1844–1858 (2007).
Nozaki, T. et al. Tight associations between transcription promoter type and epigenetic variation in histone positioning and modification. BMC Genomics 12, 1–10 (2011).
Panigrahi, A. & O’Malley, B. W. Mechanisms of enhancer action: the known and the unknown. Genome Biol. 22, 1–30 (2021).
Jin, C. et al. H3.3/H2A.Z double variant–containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat. Genet. 41, 941–945 (2009).
Lorch, Y., LaPointe, J. W. & Kornberg, R. D. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell 49, 203–210 (1987).
Canella, D., Praz, V., Reina, J. H., Cousin, P. & Hernandez, N. Defining the RNA polymerase III transcriptome: genome-wide localization of the RNA polymerase III transcription machinery in human cells. Genome Res. 20, 710–721 (2010).
Krol, A., Carbon, P., Ebel, J. & Appel, B. Xenopus tropicalis U6 snRNA genes transcribed by Pot III contain the upstream promoter elements used by pol II dependent U snRNA genes. Nucleic Acids Res. 15, 2463–2478 (1987).
Kunkel, G. R. & Pederson, T. Upstream elements required for efficient transcription of a human U6 RNA gene resemble those of U1 and U2 genes even though a different polymerase is used. Genes Dev. 2, 196–204 (1988).
Fowlkes, D. M. & Shenk, T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell 22, 405–413 (1980).
Sakonju, S., Bogenhagen, D. F. & Brown, D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5′ border of the region. Cell 19, 13–25 (1980).
Bogenhagen, D. F., Sakonju, S. & Brown, D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3′ border of the region. Cell 19, 27–35 (1980).
Das, G., Henning, D., Wright, D. & Reddy, R. Upstream regulatory elements are necessary and sufficient for transcription of a U6 RNA gene by RNA polymerase III. EMBO J. 7, 503–512 (1988).
Pieler, T., Hamm, J. & Roeder, R. G. The 5S gene internal control region is composed of three distinct sequence elements, organized as two functional domains with variable spacing. Cell 48, 91–100 (1987).
Galli, G., Hofstetter, H. & Birnstiel, M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature 294, 626–631 (1981).
Helbo, A. S., Lay, F. D., Jones, P. A., Liang, G. & Grønbæk, K. Nucleosome positioning and NDR structure at RNA polymerase III promoters. Sci. Rep. 7, 1–11 (2017).
Park, J. L. et al. Epigenetic regulation of noncoding RNA transcription by mammalian RNA polymerase III. Epigenomics 9, 171–187 (2017).
Moorefield, B., Greene, E. A. & Reeder, R. H. RNA polymerase I transcription factor Rrn3 is functionally conserved between yeast and human. Proc. Natl Acad. Sci. USA 97, 4724–4729 (2000).
Yamamoto, R. T., Nogi, Y., Dodd, J. A. & Nomura, M. RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. EMBO J. 15, 3964–3973 (1996).
Blattner, C. et al. Molecular basis of Rrn3-regulated RNA polymerase I initiation and cell growth. Genes Dev. 25, 2093–2105 (2011).
Milkereit, P. & Tschochner, H. A specialized form of RNA polymerase I, essential for initiation and growth-dependent regulation of rRNA synthesis, is disrupted during transcription. EMBO J. 17, 3692–3703 (1998).
Milkereit, P., Schultz, P. & Tschochner, H. Resolution of RNA polymerase I into dimers and monomers and their function in transcription. Biol. Chem. 378, 1433–1443 (1997).
Heiss, F. B., Daiß, J. L., Becker, P. & Engel, C. Conserved strategies of RNA polymerase I hibernation and activation. Nat. Commun. 12, 758 (2021).
Mayer, C., Bierhoff, H. & Grummt, I. The nucleolus as a stress sensor: JNK2 inactivates the transcription factor TIF-IA and down-regulates rRNA synthesis. Genes Dev. 19, 933–941 (2005).
Mayer, C., Zhao, J., Yuan, X. & Grummt, I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 18, 423–434 (2004).
Engel, C., Plitzko, J. & Cramer, P. RNA polymerase I–Rrn3 complex at 4.8 Å resolution. Nat. Commun. 7, 12129 (2016).
Pilsl, M. et al. Structure of the initiation-competent RNA polymerase I and its implication for transcription. Nat. Commun. 7, 12126 (2016).
Sadian, Y. et al. Structural insights into transcription initiation by yeast RNA polymerase I. EMBO J. 36, 2698–2709 (2017).
Engel, C. et al. Structural basis of RNA polymerase I transcription initiation. Cell 169, 120–131.e22 (2017).
Han, Y. et al. Structural mechanism of ATP-independent transcription initiation by RNA polymerase I. eLife 6, e27414 (2017).
Sadian, Y. et al. Molecular insight into RNA polymerase I promoter recognition and promoter melting. Nat. Commun. 10, 5543 (2019).
Pilsl, M. & Engel, C. Structural basis of RNA polymerase I pre-initiation complex formation and promoter melting. Nat. Commun. 11, 1206 (2020).
Comai, L. et al. Reconstitution of transcription factor SL1: exclusive binding of TBP by SL1 or TFIID subunits. Science 266, 1966–1972 (1994).
Bedwell, G. J., Appling, F. D., Anderson, S. J. & Schneider, D. A. Efficient transcription by RNA polymerase I using recombinant core factor. Gene 492, 94–99 (2012).
Baudin, F. et al. Mechanism of RNA polymerase I selection by transcription factor UAF. Preprint at bioRxiv https://doi.org/10.1101/2022.02.10.479882 (2022).
Panov, K. I., Friedrich, J. K. & Zomerdijk, J. C. B. M. A step subsequent to preinitiation complex assembly at the ribosomal RNA gene promoter is rate limiting for human RNA polymerase I-dependent transcription. Mol. Cell. Biol. 21, 2641–2649 (2001).
Hahn, S. & Young, E. T. Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators. Genetics 189, 705 (2011).
Sainsbury, S., Bernecky, C. & Cramer, P. Structural basis of transcription initiation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 16, 129–143 (2015).
Buratowski, S., Hahn, S., Guarente, L. & Sharp, P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell 56, 549–561 (1989).
Soutourina, J. Transcription regulation by the Mediator complex. Nat. Rev. Mol. Cell Biol. 19, 262–274 (2018).
Plaschka, C. et al. Architecture of the RNA polymerase II–Mediator core initiation complex. Nature 518, 376–380 (2015).
Plaschka, C. et al. Transcription initiation complex structures elucidate DNA opening. Nature 533, 353–358 (2016).
Schilbach, S. et al. Structures of transcription pre-initiation complex with TFIIH and Mediator. Nature 551, 204–209 (2017).
Tsai, K.-L. et al. Mediator structure and rearrangements required for holoenzyme formation. Nature 544, 196–201 (2017).
Dienemann, C., Schwalb, B., Schilbach, S. & Cramer, P. Promoter distortion and opening in the RNA polymerase II cleft. Mol. Cell 73, 97–106.e4 (2019).
Schilbach, S., Aibara, S., Dienemann, C., Grabbe, F. & Cramer, P. Structure of RNA polymerase II pre-initiation complex at 2.9 Å defines initial DNA opening. Cell 184, 4064–4072.e28 (2021).
Yang, C. et al. Structural visualization of de novo transcription initiation by Saccharomyces cerevisiae RNA polymerase II. Mol. Cell 82, 660–676.e9 (2022).
He, Y. et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533, 359–365 (2016).
Abdella, R. et al. Structure of the human Mediator-bound transcription preinitiation complex. Science 372, 52–56 (2021).
Chen, X. et al. Structural insights into preinitiation complex assembly on core promoters. Science 372, eaba8490 (2021).
Rengachari, S., Schilbach, S., Aibara, S., Dienemann, C. & Cramer, P. Structure of the human Mediator–RNA polymerase II pre-initiation complex. Nature 594, 129–133 (2021).
Aibara, S., Schilbach, S. & Cramer, P. Structures of mammalian RNA polymerase II pre-initiation complexes. Nature 594, 124–128 (2021).
Chen, X. et al. Structures of the human Mediator and Mediator-bound preinitiation complex. Science 372, eabg0635 (2021).
Cianfrocco, M. A. et al. Human TFIID binds to core promoter DNA in a reorganized structural state. Cell 152, 120–131 (2013).
Patel, A. B. et al. Structure of human TFIID and mechanism of TBP loading onto promoter DNA. Science 362, eaau8872 (2018).
Kim, Y., Geiger, J. H., Hahn, S. & Sigler, P. B. Crystal structure of a yeast TBP/TATA-box complex. Nature 365, 512–520 (1993).
Chen, F. X., Smith, E. R. & Shilatifard, A. Born to run: control of transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 19, 464–478 (2018).
Fisher, R. P. & Morgan, D. O. A novel cyclin associates with M015/CDK7 to form the CDK-activating kinase. Cell 78, 713–724 (1994).
Joo, Y. J. et al. Downstream promoter interactions of TFIID TAFs facilitate transcription reinitiation. Genes Dev. 31, 2162–2174 (2017).
Yudkovsky, N., Ranish, J. A. & Hahn, S. A transcription reinitiation intermediate that is stabilized by activator. Nature 408, 225–229 (2000).
Baek, I., Friedman, L. J., Gelles, J. & Buratowski, S. Single-molecule studies reveal branched pathways for activator-dependent assembly of RNA polymerase II pre-initiation complexes. Mol. Cell 81, 3576–3588.e6 (2021).
Dergai, O. & Hernandez, N. How to recruit the correct RNA polymerase? Lessons from snRNA genes. Trends Genet. 35, 457–469 (2019).
Sadowski, C. L., Henry, R. W., Lobo, S. M. & Hernandez, N. Targeting TBP to a non-TATA box cis-regulatory element: a TBP-containing complex activates transcription from snRNA promoters through the PSE. Genes Dev. 7, 1535–1548 (1993).
Dergai, O. et al. Mechanism of selective recruitment of RNA polymerases II and III to snRNA gene promoters. Genes Dev. 32, 711–722 (2018).
Kim, M. K. et al. Assembly of SNAPc, Bdp1, and TBP on the U6 snRNA gene promoter in Drosophila melanogaster. Mol. Cell. Biol. https://doi.org/10.1128/MCB.00641-19 (2020).
Gouge, J. et al. Redox signaling by the RNA polymerase III TFIIB-related factor Brf2. Cell 163, 1375–1387 (2015).
Gouge, J. et al. Molecular mechanisms of Bdp1 in TFIIIB assembly and RNA polymerase III transcription initiation. Nat. Commun. 8, 130 (2017).
Knutson, B. A. & Hahn, S. Yeast Rrn7 and human TAF1B are TFIIB-related RNA polymerase I general transcription factors. Science 333, 1637–1640 (2011).
Colbert, T. & Hahn, S. A yeast TFIIB-related factor involved in RNA polymerase III transcription. Genes Dev. 6, 1940–1949 (1992).
Vannini, A. & Cramer, P. Conservation between the RNA polymerase I, II, and III transcription initiation machineries. Mol. Cell 45, 439–446 (2012).
Wang, Z. & Roeder, R. G. Three human RNA polymerase III-specific subunits form a subcomplex with a selective function in specific transcription initiation. Genes Dev. 11, 1315–1326 (1997).
Brun, I., Sentenac, A. & Werner, M. Dual role of the C34 subunit of RNA polymerase III in transcription initiation. EMBO J. 16, 5730–5741 (1997).
Landrieux, E. et al. A subcomplex of RNA polymerase III subunits involved in transcription termination and reinitiation. EMBO J. 25, 118–128 (2006).
Kassavetis, G. A., Prakash, P. & Shim, E. The C53/C37 subcomplex of RNA polymerase III lies near the active site and participates in promoter opening. J. Biol. Chem. 285, 2695–2706 (2010).
Wu, C.-C., Lin, Y.-C. & Chen, H.-T. The TFIIF-like Rpc37/53 dimer lies at the center of a protein network to connect TFIIIC, Bdp1, and the RNA polymerase III active center. Mol. Cell. Biol. 31, 2715–2728 (2011).
Ducrot, C. et al. Reconstitution of the yeast RNA polymerase III transcription system with all recombinant factors. J. Biol. Chem. 281, 11685–11692 (2006).
Kassavetis, G. A. et al. The role of the TATA-binding protein in the assembly and function of the multisubunit yeast RNA polymerase III transcription factor, TFIIIB. Cell 71, 1055–1064 (1992).
Kassavetis, G. A., Braun, B. R., Nguyen, L. H. & Geiduschek, E. P. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell 60, 235–245 (1990).
Cieśla, M., Skowronek, E. & Boguta, M. Function of TFIIIC, RNA polymerase III initiation factor, in activation and repression of tRNA gene transcription. Nucleic Acids Res. 46, 9444–9455 (2018).
Male, G. et al. Architecture of TFIIIC and its role in RNA polymerase III pre-initiation complex assembly. Nat. Commun. 6, 7387 (2015).
Vorländer, M. K. et al. Structure of the TFIIIC subcomplex τA provides insights into RNA polymerase III pre-initiation complex formation. Nat. Commun. 11, 4905 (2020).
Willis, I. M. & Moir, R. D. Signaling to and from the RNA polymerase III transcription and processing machinery. Annu. Rev. Biochem. 87, 75–100 (2018).
Vorländer, M. K. et al. Structural basis for RNA polymerase III transcription repression by Maf1. Nat. Struct. Mol. Biol. 27, 229–232 (2020).
Tafur, L. et al. Molecular structures of transcribing RNA polymerase I. Mol. Cell 64, 1135–1143 (2016).
Neyer, S. et al. Structure of RNA polymerase I transcribing ribosomal DNA genes. Nature 540, 607–610 (2016).
McNamar, R., Abu-Adas, Z., Rothblum, K., Knutson, B. A. & Rothblum, L. I. Conditional depletion of the RNA polymerase I subunit PAF53 reveals that it is essential for mitosis and enables identification of functional domains. J. Biol. Chem. 294, 19907–19922 (2019).
Tafur, L. et al. The cryo-EM structure of a 12-subunit variant of RNA polymerase I reveals dissociation of the A49-A34.5 heterodimer and rearrangement of subunit A12.2. eLife 8, e43204 (2019).
Scull, C. E., Lucius, A. L. & Schneider, D. A. The N-terminal domain of the A12.2 subunit stimulates RNA polymerase I transcription elongation. Biophys. J. 120, 1883–1893 (2021).
Wada, T. et al. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12, 343–356 (1998).
Schneider, D. A. et al. RNA polymerase II elongation factors Spt4p and Spt5p play roles in transcription elongation by RNA polymerase I and rRNA processing. Proc. Natl Acad. Sci. USA 103, 12707–12712 (2006).
Viktorovskaya, O. V., Appling, F. D. & Schneider, D. A. Yeast transcription elongation factor Spt5 associates with RNA polymerase I and RNA polymerase II directly. J. Biol. Chem. 286, 18825–18833 (2011).
Anderson, S. J. et al. The transcription elongation factor Spt5 influences transcription by RNA polymerase I positively and negatively. J. Biol. Chem. 286, 18816–18824 (2011).
Huffines, A. K., Edwards, Y. J. K. & Schneider, D. A. Spt4 promotes Pol I processivity and transcription elongation. Genes 12, 413 (2021).
Zhang, Y., Sikes, M. L., Beyer, A. L. & Schneider, D. A. The Paf1 complex is required for efficient transcription elongation by RNA polymerase I. Proc. Natl Acad. Sci. USA 106, 2153–2158 (2009).
Stefanovsky, V., Langlois, F., Gagnon-Kugler, T., Rothblum, L. I. & Moss, T. Growth factor signaling regulates elongation of RNA polymerase I transcription in mammals via UBF phosphorylation and r-chromatin remodeling. Mol. Cell 21, 629–639 (2006).
Fath, S., Kobor, M. S., Philippi, A., Greenblatt, J. & Tschochner, H. Dephosphorylation of RNA polymerase I by Fcp1p is required for efficient rRNA synthesis. J. Biol. Chem. 279, 25251–25259 (2004).
Turowski, T. W. et al. Nascent transcript folding plays a major role in determining RNA polymerase elongation rates. Mol. Cell 79, 488–503.e11 (2020).
Schneider, D. A. et al. Transcription elongation by RNA polymerase I is linked to efficient rRNA processing and ribosome assembly. Mol. Cell 26, 217–229 (2007).
Osheim, Y. N. et al. Pre-18S ribosomal RNA is structurally compacted into the SSU processome prior to being cleaved from nascent transcripts in Saccharomyces cerevisiae. Mol. Cell 16, 943–954 (2004).
Miller, O. L. & Beatty, B. R. Visualization of nucleolar genes. Science 164, 955–957 (1969).
Albert, B., Perez-Fernandez, J., Léger-Silvestre, I. & Gadal, O. Regulation of ribosomal RNA production by RNA polymerase I: does elongation come first? Genet. Res. Int. 2012, 1–13 (2012).
El Hage, A., French, S. L., Beyer, A. L. & Tollervey, D. Loss of topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev. 24, 1546–1558 (2010).
Yamaguchi, Y. et al. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell 97, 41–51 (1999).
Vos, S. M., Farnung, L., Urlaub, H. & Cramer, P. Structure of paused transcription complex Pol II–DSIF–NELF. Nature 560, 601–606 (2018).
Vos, S. M. et al. Structure of activated transcription complex Pol II–DSIF–PAF–SPT6. Nature 560, 607–612 (2018).
Bernecky, C., Plitzko, J. M. & Cramer, P. Structure of a transcribing RNA polymerase II–DSIF complex reveals a multidentate DNA–RNA clamp. Nat. Struct. Mol. Biol. 24, 809–815 (2017).
Cheung, A. C. M. & Cramer, P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature 471, 249–253 (2011).
Vos, S. M., Farnung, L., Linden, A., Urlaub, H. & Cramer, P. Structure of complete Pol II–DSIF–PAF–SPT6 transcription complex reveals RTF1 allosteric activation. Nat. Struct. Mol. Biol. 27, 668–677 (2020).
Chen, Y. et al. Allosteric transcription stimulation by RNA polymerase II super elongation complex. Mol. Cell 81, 3386–3399.e10 (2021).
Rossi, M. J. et al. A high-resolution protein architecture of the budding yeast genome. Nature 592, 309–314 (2021).
Kujirai, T. et al. Structural basis of the nucleosome transition during RNA polymerase II passage. Science 362, 595–598 (2018).
Farnung, L., Vos, S. M. & Cramer, P. Structure of transcribing RNA polymerase II-nucleosome complex. Nat. Commun. 9, 5432 (2018).
Ehara, H. et al. Structural insight into nucleosome transcription by RNA polymerase II with elongation factors. Science 363, 744–747 (2019).
Farnung, L., Ochmann, M., Engeholm, M. & Cramer, P. Structural basis of nucleosome transcription mediated by Chd1 and FACT. Nat. Struct. Mol. Biol. 28, 382–387 (2021).
Orphanides, G., LeRoy, G., Chang, C.-H., Luse, D. S. & Reinberg, D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92, 105–116 (1998).
Jeronimo, C. et al. FACT is recruited to the +1 nucleosome of transcribed genes and spreads in a Chd1-dependent manner. Mol. Cell 81, 3542–3559.e11 (2021).
Cho, E.-J., Takagi, T., Moore, C. R. & Buratowski, S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 11, 3319–3326 (1997).
Wahl, M. C., Will, C. L. & Lührmann, R. The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718 (2009).
Zhang, S. et al. Structure of a transcribing RNA polymerase II–U1 snRNP complex. Science 371, 305–309 (2021).
Zhang, J., Cavallaro, M. & Hebenstreit, D. Timing RNA polymerase pausing with TV-PRO-seq. Cell Rep. Methods 1, 100083 (2021).
Alic, N. et al. Selectivity and proofreading both contribute significantly to the fidelity of RNA polymerase III transcription. Proc. Natl Acad. Sci. USA 104, 10400–10405 (2007).
French, S. L. et al. Visual analysis of the yeast 5S rRNA gene transcriptome: Regulation and role of La protein. Mol. Cell. Biol. 28, 4576–4587 (2008).
Jarrous, N., Mani, D. & Ramanathan, A. Coordination of transcription and processing of tRNA. FEBS J. https://doi.org/10.1111/febs.15904 (2021).
Maraia, R. J., Mattijssen, S., Cruz-Gallardo, I. & Conte, M. R. The La and related RNA-binding proteins (LARPs): structures, functions, and evolving perspectives. Wiley Interdiscip. Rev. RNA 8, e1430 (2017).
Serruya, R. et al. Human RNase P ribonucleoprotein is required for formation of initiation complexes of RNA polymerase III. Nucleic Acids Res. 43, 5442–5450 (2015).
Henras, A. K. et al. Biochemical and genomic analysis of substrate recognition by the double-stranded RNA binding domain of yeast RNase III. RNA 11, 1225–1237 (2005).
Mekhail, K., Seebacher, J., Gygi, S. P. & Moazed, D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature 456, 667–670 (2008).
Takeuchi, Y., Horiuchi, T. & Kobayashi, T. Transcription-dependent recombination and the role of fork collision in yeast rDNA. Genes Dev. 17, 1497–1506 (2003).
Wu, H., Henras, A., Chanfreau, G. & Feigon, J. Structural basis for recognition of the AGNN tetraloop RNA fold by the double-stranded RNA-binding domain of Rnt1p RNase III. Proc. Natl Acad. Sci. USA 101, 8307–8312 (2004).
El Hage, A., Koper, M., Kufel, J. & Tollervey, D. Efficient termination of transcription by RNA polymerase I requires the 5′ exonuclease Rat1 in yeast. Genes Dev. 22, 1069–1081 (2008).
Reeder, R. H., Guevara, P. & Roan, J. G. Saccharomyces cerevisiae RNA polymerase I terminates transcription at the Reb1 terminator in vivo. Mol. Cell. Biol. 19, 7369–7376 (1999).
Lang, W. H. & Reeder, R. H. The REB1 site is an essential component of a terminator for RNA polymerase I in Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 649–658 (1993).
Reiter, A. et al. The Reb1-homologue Ydr026c/Nsi1 is required for efficient RNA polymerase I termination in yeast. EMBO J. 31, 3480–3493 (2012).
Lang, W. H. & Reeder, R. H. Transcription termination of RNA polymerase I due to a T-rich element interacting with Reb1p. Proc. Natl Acad. Sci. USA 92, 9781–9785 (1995).
Jaiswal, R. et al. Functional architecture of the Reb1-Ter complex of Schizosaccharomyces pombe. Proc. Natl Acad. Sci. USA 113, E2267–E2276 (2016).
Jeong, S. W., Lang, W. H. & Reeder, R. H. The yeast transcription terminator for RNA polymerase I is designed to prevent polymerase slippage. J. Biol. Chem. 271, 16104–16110 (1996).
Prescott, E. M. et al. Transcriptional termination by RNA polymerase I requires the small subunit Rpa12p. Proc. Natl Acad. Sci. USA 101, 6068–6073 (2004).
Grummt, I., Rosenbauer, H., Niedermeyer, I., Maier, U. & Öhrlein, A. A repeated 18 bp sequence motif in the mouse rDNA spacer mediates binding of a nuclear factor and transcription termination. Cell 45, 837–846 (1986).
Jansa, P., Mason, S. W., Hoffmann-Rohrer, U. & Grummt, I. Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J. 17, 2855–2864 (1998).
Smid, A., Finsterer, M. & Grummt, I. Limited proteolysis unmasks specific DNA-binding of the murine RNA polymerase I-specific transcription termination factor TTFI. J. Mol. Biol. 227, 635–647 (1992).
Akamatsu, Y. & Kobayashi, T. The human RNA polymerase I transcription terminator complex acts as a replication fork barrier that coordinates the progress of replication with rRNA transcription activity. Mol. Cell. Biol. 35, 1871–1881 (2015).
Porrua, O. & Libri, D. Transcription termination and the control of the transcriptome: why, where and how to stop. Nat. Rev. Mol. Cell Biol. 16, 190–202 (2015).
Eaton, J. D. & West, S. Termination of transcription by RNA polymerase II: BOOM! Trends Genet. 36, 664–675 (2020).
Kumar, A., Clerici, M., Muckenfuss, L. M., Passmore, L. A. & Jinek, M. Mechanistic insights into mRNA 3′-end processing. Curr. Opin. Struct. Biol. 59, 143–150 (2019).
Lunde, B. M. et al. Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat. Struct. Mol. Biol. 17, 1195–1201 (2010).
Kim, M. et al. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432, 517–522 (2004).
West, S., Gromak, N. & Proudfoot, N. J. Human 5’→3’ exonuclease Xrn2 promotes transcription termination at co-transcriptional cleavage sites. Nature 432, 522–525 (2004).
Kamieniarz-Gdula, K. et al. Selective roles of vertebrate PCF11 in premature and full-length transcript termination. Mol. Cell 74, 158–172.e9 (2019).
Cortazar, M. A. et al. Control of RNA Pol II speed by PNUTS-PP1 and Spt5 dephosphorylation facilitates termination by a “sitting duck torpedo” mechanism. Mol. Cell 76, 896–908.e4 (2019).
Parua, P. K. et al. A Cdk9–PP1 switch regulates the elongation–termination transition of RNA polymerase II. Nature 558, 460–464 (2018).
Kecman, T. et al. Elongation/Termination factor exchange mediated by PP1 phosphatase orchestrates transcription termination. Cell Rep. 25, 259–269.e5 (2018).
Steinmetz, E. J., Conrad, N. K., Brow, D. A. & Corden, J. L. RNA-binding protein Nrd1 directs poly(A)-independent 3′-end formation of RNA polymerase II transcripts. Nature 413, 327–331 (2001).
Vasiljeva, L., Kim, M., Mutschler, H., Buratowski, S. & Meinhart, A. The Nrd1–Nab3–Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat. Struct. Mol. Biol. 15, 795–804 (2008).
Porrua, O. & Libri, D. A bacterial-like mechanism for transcription termination by the Sen1p helicase in budding yeast. Nat. Struct. Mol. Biol. 20, 884–891 (2013).
Leonaitė, B. et al. Sen1 has unique structural features grafted on the architecture of the Upf1-like helicase family. EMBO J. 36, 1590–1604 (2017).
Han, Z. et al. Termination of non-coding transcription in yeast relies on both an RNA Pol II CTD interaction domain and a CTD-mimicking region in Sen1. EMBO J. 39, e101548 (2020).
Han, Z., Libri, D. & Porrua, O. Biochemical characterization of the helicase Sen1 provides new insights into the mechanisms of non-coding transcription termination. Nucleic Acids Res. 45, 1355–1370 (2017).
Baillat, D. et al. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123, 265–276 (2005).
Yamamoto, J. et al. DSIF and NELF interact with Integrator to specify the correct post-transcriptional fate of snRNA genes. Nat. Commun. 5, 4263 (2014).
Zheng, H. et al. Identification of Integrator-PP2A complex (INTAC), an RNA polymerase II phosphatase. Science 370, eabb5872 (2020).
Fianu, I. et al. Structural basis of Integrator-mediated transcription regulation. Science 374, 883–887 (2021).
Cozzarelli, N. R., Gerrard, S. P., Schlissel, M., Brown, D. D. & Bogenhagen, D. F. Purified RNA polymerase III accurately and efficiently terminates transcription of 5S RNA genes. Cell 34, 829–835 (1983).
Dieci, G. & Sentenac, A. Facilitated recycling pathway for RNA polymerase III. Cell 84, 245–252 (1996).
James, P. & Hall, B. D. Ret1-1, a yeast mutant affecting transcription termination by RNA polymerase III. Genetics 125, 293–303 (1990).
Shaaban, S. A., Krupp, B. M. & Hall, B. D. Termination-altering mutations in the second-largest subunit of yeast RNA polymerase III. Mol. Cell. Biol. 15, 1467–1478 (1995).
Rijal, K. & Maraia, R. J. Active center control of termination by RNA polymerase III and tRNA gene transcription levels in vivo. PLoS Genet. 12, e1006253 (2016).
Rijal, K. & Maraia, R. J. RNA polymerase III mutants in TFIIFα-like C37 that cause terminator readthrough with no decrease in transcription output. Nucleic Acids Res. 41, 139–155 (2013).
Arimbasseri, A. G. & Maraia, R. J. Distinguishing core and holoenzyme mechanisms of transcription termination by RNA polymerase III. Mol. Cell. Biol. 33, 1571–1581 (2013).
Arimbasseri, A. G. & Maraia, R. J. Mechanism of transcription termination by RNA polymerase III utilizes a non-template strand sequence-specific signal element. Mol. Cell 58, 1124–1132 (2015).
Iben, J. R. et al. Point mutations in the Rpb9-homologous domain of Rpc11 that impair transcription termination by RNA polymerase III. Nucleic Acids Res. 39, 6100–6113 (2011).
Hou, H. et al. Structural insights into RNA polymerase III-mediated transcription termination through trapping poly-deoxythymidine. Nat. Commun. 12, 6135 (2021).
Girbig, M. et al. Architecture of the yeast Pol III pre-termination complex and pausing mechanism on poly-dT termination signals. bioRxiv https://doi.org/10.1101/2022.02.28.482286 (2022).
Martin, F. H. & Tinoco, I. DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 8, 2295–2300 (1980).
Mishra, S., Hasan, S. H., Sakhawala, R. M., Chaudhry, S. & Maraia, R. J. Mechanism of RNA polymerase III termination-associated reinitiation-recycling conferred by the essential function of the N terminal-and-linker domain of the C11 subunit. Nat. Commun. 12, 5900 (2021).
Rivosecchi, J. et al. Senataxin homologue Sen1 is required for efficient termination of RNA polymerase III transcription. EMBO J. 38, e101955 (2019).
Xie, J. et al. An integrated model for termination of RNA polymerase III transcription. bioRxiv https://doi.org/10.1101/2021.08.27.457903 (2021).
Ferrari, R., Rivetti, C., Acker, J. & Dieci, G. Distinct roles of transcription factors TFIIIB and TFIIIC in RNA polymerase III transcription reinitiation. Proc. Natl Acad. Sci. USA 101, 13442–13447 (2004).
Steffan, J. S., Keys, D. A., Vu, L. & Nomura, M. Interaction of TATA-binding protein with upstream activation factor is required for activated transcription of ribosomal DNA by RNA polymerase I in Saccharomyces cerevisiae in vivo. Mol. Cell. Biol. 18, 3752–3761 (1998).
Bell, S. P., Learned, R. M., Jantzen, H.-M. & Tjian, R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science 241, 1192–1197 (1988).
Timmers, H. T. M. SAGA and TFIID: Friends of TBP drifting apart. Biochim. Biophys. Acta - Gene Regul. Mech. 1864, 194604 (2021).
Acknowledgements
This work was supported by the European Molecular Biology Laboratory (M.G., A.D.M. and C.W.M.) and the European Molecular Biology Laboratory International PhD Programme (M.G. and A.D.M.). The authors thank F. Baudin for feedback on the manuscript. M.G. acknowledges support from a Boehringer Ingelheim Fonds PhD fellowship. The authors apologize to those authors whose work has not been included because of space limitations.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Shun-ichi Sekine, Dong Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Active site
-
A common term for the catalytic centre of RNA polymerases.
- Stalk
-
A mobile structural element found in archaeal and eukaryotic RNA polymerases that is composed of one or two subunits and functions in transcription initiation.
- Alu elements
-
Short, primate-specific transposons transcribed by RNA polymerase III (Pol III) or Pol II.
- Microsatellites
-
Tandem repeats of 1–6 bp that are often used as genetic markers.
- Transposons
-
Mobile DNA elements that can relocate within the genome.
- Spacer promoters
-
Sequences in the spacer regions of ribosomal DNA repeats adjacent to ribosomal DNA promoters, with which they share a high degree of similarity.
- Proximal terminators
-
Terminator elements directly upstream of ribosomal RNA gene promoters which prevent readthrough of polymerases from the intergenic spacer into the promoter that is bound by the stable RNA polymerase I (Pol I) preinitiation complex.
- R-loops
-
Three-stranded RNA–DNA hybrid structures that form when a transcribed RNA invades the double-stranded DNA and hybridizes with its complementary template strand, thereby rendering the non-template single-stranded.
- Terminator sequences
-
Nucleic acid sequences that mark the end of a gene and stimulate transcription termination.
- Activator-dependent promoter
-
Promoter element that exerts high and specific promoter activity only when accompanied by additional motifs bound by other activators.
- Stably positioned nucleosomes
-
Nucleosomes that are stably (strongly) positioned in the genome across many cells.
- Dynamic nucleosomes
-
Nucleosomes whose positions are fuzzy owing to, for example, DNA sequences or activity of chromatin remodellers.
- Dock
-
A small protein domain found in subunit A190 of RNA polymerase I (Pol I), subunit RPB1 of Pol II and subunit C160 of Pol III, which serves as a binding platform for transcription initiation factors.
- Clamp domain
-
A mobile protein domain found in all DNA-dependent RNA polymerases that adopts an ‘open’ conformation in DNA-unbound polymerases, but closes upon DNA binding, and thereby participates in various steps during the transcription process.
- Ribosome processomes
-
Large ribonucleoprotein complexes formed between the pre-ribosomal RNA, ribosomal proteins and RNA-processing factors during the process of ribosome maturation.
- Funnel
-
Structural element of RNA polymerases that serves as the entry channel for nucleoside triphosphates and the exit channel for the RNA 3′ end, which is expelled from the active site upon polymerase backtracking.
- Backtracking
-
RNA polymerase backward movement owing to stalling (for example, due to nucleotide misincorporation), which requires intrinsic or extrinsic RNA cleavage to restore transcript elongation.
Rights and permissions
About this article
Cite this article
Girbig, M., Misiaszek, A.D. & Müller, C.W. Structural insights into nuclear transcription by eukaryotic DNA-dependent RNA polymerases. Nat Rev Mol Cell Biol 23, 603–622 (2022). https://doi.org/10.1038/s41580-022-00476-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-022-00476-9
This article is cited by
-
KLF7 regulates super-enhancer-driven IGF2BP2 overexpression to promote the progression of head and neck squamous cell carcinoma
Journal of Experimental & Clinical Cancer Research (2024)
-
Structural basis of exoribonuclease-mediated mRNA transcription termination
Nature (2024)
-
A cryo-EM structure of KTF1-bound polymerase V transcription elongation complex
Nature Communications (2023)
-
The conserved RNA-binding protein Seb1 promotes cotranscriptional ribosomal RNA processing by controlling RNA polymerase I progression
Nature Communications (2023)
-
Orphan quality control by an SCF ubiquitin ligase directed to pervasive C-degrons
Nature Communications (2023)