Abstract
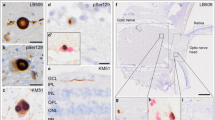
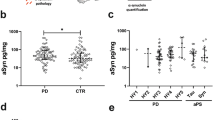
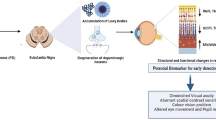
The pathognomonic hallmark of Parkinson’s disease (PD), α-synuclein, has been observed in the retina of PD patients. We investigated whether biomarkers in the tears and retinal microvascular changes associate with PD risk and progression. This prospective study enrolled 49 PD patients and 45 age-matched healthy controls. The α-synuclein and neurofilament light chain (NfL) levels were measured using an electrochemiluminescence immunoassay. Retinal vessel density was assessed using optical coherence tomography angiography (OCT-A). The Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and Mini-Mental State Examination score were used to assess motor and cognitive progression. The α-synuclein and NfL levels in the tears were higher in PD patients than in controls (α-synuclein: 55.49 ± 8.12 pg/mL vs. 31.71 ± 3.25 pg/mL, P = 0.009; NfL: 2.89 ± 0.52 pg/mL vs. 1.47 ± 0.23 pg/mL, P = 0.02). The vessel densities in the deep plexus of central macula and the radial peripapillary capillary layer of disc region were lower in PD patients with moderate-stage compared with early-stage PD (P < 0.05). The accuracy of predicting PD occurrence using age and sex alone (area under the curve [AUC] 0.612) was significantly improved by adding α-synuclein and NfL levels and retinal vascular densities (AUC 0.752, P = 0.001). After a mean follow-up of 1.5 ± 0.3 years, the accuracy of predicting motor or cognitive progression using age, sex, and baseline motor severity as a basic model was increased by incorporating retinal microvascular and biofluid markers as a full model (P = 0.001). Our results showed that retinal microvascular densities combined with α-synuclein and NfL levels in tears are associated with risk and progression of PD.




Similar content being viewed by others
References
Collaborators GBDPsD. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–53.
Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1615–22.
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44.
Armstrong RA. Oculo-visual dysfunction in Parkinson’s disease. J Parkinsons Dis. 2015;5:715–26.
Frederick JM, Rayborn ME, Laties AM, Lam DM, Hollyfield JG. Dopaminergic neurons in the human retina. J Comp Neurol. 1982;210:65–79.
Harnois C, Di Paolo T. Decreased dopamine in the retinas of patients with Parkinson’s disease. Invest Ophthalmol Vis Sci. 1990;31:2473–5.
Ortuno-Lizaran I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018;33:1315–24.
Lee JY, Ahn J, Oh S, Shin JY, Kim YK, Nam H, Jeon B. Retina thickness as a marker of neurodegeneration in prodromal lewy body disease. Mov Disord. 2020;35:349–54.
Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson’s disease. Brain. 2009;132:1128–45.
Moschos MM, Gonidakis F, Varsou E, Markopoulos I, Rouvas A, Ladas I, Papadimitriou GN. Anatomical and functional impairment of the retina and optic nerve in patients with anorexia nervosa without vision loss. Br J Ophthalmol. 2011;95:1128–33.
Garcia-Martin E, Satue M, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Fernandez J, Pablo LE. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology. 2012;119:2161–7.
Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Pablo LE, Fernandez FJ. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye (Lond). 2013;27:507–14.
Huang L, Wang Y, Zhang R. Intravenous thrombolysis in patients with central retinal artery occlusion: a systematic review and meta-analysis. J Neurol. 2021. https://doi.org/10.1007/s00415-021-10838-6.
MacCormick IJ, Czanner G, Faragher B. Developing retinal biomarkers of neurological disease: an analytical perspective. Biomark Med. 2015;9:691–701.
Hase Y, Ding R, Harrison G, Hawthorne E, King A, Gettings S, Platten C, Stevenson W, Craggs LJL, Kalaria RN. White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathol Commun. 2019;7:16. https://doi.org/10.1186/s40478-019-0666-x.
Elahi FM, Ashimatey SB, Bennett DJ, Walters SM, La Joie R, Jiang X, Wolf A, Cobigo Y, Staffaroni AM, Rosen HJ, Miller BL, Rabinovici GD, Kramer JH, Green AJ, Kashani AH. Retinal imaging demonstrates reduced capillary density in clinically unimpaired APOE ε4 gene carriers. Alzheimers Dement (Amst). 2021;13(1):e12181. https://doi.org/10.1002/dad2.12181.
Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, Green CR, Nicholson LF. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013;23:154–64.
Shi C, Chen Y, Kwapong WR, Tong Q, Wu S, Zhou Y, Miao H, Shen M, Ye H. Characterization by fractal dimension analysis of the retinal capillary network in Parkinson disease. Retina. 2020;40:1483–91.
Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, Yoon SP, Polascik BW, Scott BL, Grewal DS, Fekrat S. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol. 2021;139:182–8.
Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–77.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4.
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Poewe W. Clinical measures of progression in Parkinson’s disease. Mov Disord. 2009;24(Suppl 2):S671-676.
Lin CH, Li CH, Yang KC, Lin FJ, Wu CC, Chieh JJ, Chiu MJ. Blood NfL: A biomarker for disease severity and progression in Parkinson disease. Neurology. 2019;93:e1104–11.
Maass F, Rikker S, Dambeck V, Warth C, Tatenhorst L, Csoti I, Schmitz M, Zerr I, Leha A, Bahr M, Lingor P. Increased alpha-synuclein tear fluid levels in patients with Parkinson’s disease. Sci Rep. 2020;10:8507.
Hamm-Alvarez SF, Janga SR, Edman MC, Feigenbaum D, Freire D, Mack WJ, Okamoto CT, Lew MF. Levels of oligomeric alpha-Synuclein in reflex tears distinguish Parkinson’s disease patients from healthy controls. Biomark Med. 2019;13:1447–57.
Hamm-Alvarez SF, Okamoto CT, Janga SR, Feigenbaum D, Edman MC, Freire D, Shah M, Ghanshani R, Mack WJ, Lew MF. Oligomeric alpha-synuclein is increased in basal tears of Parkinson’s patients. Biomark Med. 2019;13:941–52.
Atik A, Stewart T, Zhang J. Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol. 2016;26:410–8.
Costa OR, Verhaeghen K, Roels S, Stange G, Ling Z, Pipeleers D, Gorus FK, Martens GA. An analytical comparison of three immunoassay platforms for subpicomolar detection of protein biomarker GAD65. PLoS ONE. 2018;13:e0193670.
Lee SJ, Desplats P, Lee HJ, Spencer B, Masliah E. Cell-to-cell transmission of alpha-synuclein aggregates. Methods Mol Biol. 2012;849:347–59.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Fyfe I. Ultrasensitive assay raises hope of plasma PD marker. Nat Rev Neurol. 2019;15:186–7.
Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233:183–98.
Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91:56–66.
Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFLG, Alvarez-Cermeno JC, Andreasson U, Axelsson M, Backstrom DC, Bartos A, Bjerke M, Blennow K, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 2019;76:1035–48.
Mollenhauer B, Dakna M, Kruse N, Galasko D, Foroud T, Zetterberg H, Schade S, Gera RG, Wang W, Gao F, Frasier M, Chahine LM, Coffey CS, et al. Validation of serum neurofilament light chain as a biomarker of Parkinson’s disease Progression. Mov Disord. 2020;35:1999–2008.
Backstrom D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L, Lenfeldt N. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology. 2020;95:e827–38.
Gijs M, Ramakers I, Visser PJ, Verhey FRJ, van de Waarenburg MPH, Schalkwijk CG, Nuijts R, Webers CAB. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci Rep. 2021;11:22675.
Comoglu SS, Guven H, Acar M, Ozturk G, Kocer B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson’s disease. Neurosci Lett. 2013;553:63–7.
Salvisberg C, Tajouri N, Hainard A, Burkhard PR, Lalive PH, Turck N. Exploring the human tear fluid: discovery of new biomarkers in multiple sclerosis. Proteomics Clin Appl. 2014;8:185–94.
Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, Lu F. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:4115–22.
Zhang Y, Shi C, Chen Y, Wang W, Huang S, Han Z, Lin X, Lu F, Shen M. Retinal Structural and microvascular alterations in different acute ischemic stroke subtypes. J Ophthalmol. 2020;5:2422. https://doi.org/10.1155/2020/8850309.
Bhanushali D, Anegondi N, Gadde SG, Srinivasan P, Chidambara L, Yadav NK, Sinha RA. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:519–25.
Turski CA, Turski GN, Faber J, Teipel SJ, Holz FG, Klockgether T, Finger RP. Microvascular breakdown due to retinal neurodegeneration in ataxias. Mov Disord. 2022;37:162–70.
Price DL, Rockenstein E, Mante M, Adame A, Overk C, Spencer B, Duong-Polk KX, Bonhaus D, Lindsey J, Masliah E. Longitudinal live imaging of retinal alpha-synuclein::GFP deposits in a transgenic mouse model of Parkinson’s Disease/Dementia with Lewy Bodies. Sci Rep. 2016;6:29523.
Acknowledgements
The authors thank all the participants in this study. They also thank the staff of the Second Core Laboratory, Department of Medical Research, National Taiwan University Hospital for their technical support of the study.
Funding
The authors are grateful to the funding support from the Academia Sinica (AS-HLGC-110–03) and National Taiwan University Hospital (111-UN0036).
Author information
Authors and Affiliations
Contributions
Conceptualization: CW Lin and CH Lin; data curation: CW Lin, TT Lai, SJ Chen, and CH Lin; formal analysis: CW Lin, TT Lai, and SJ Chen; funding acquisition: CH Lin. investigation: CW Lin, TT Lai, and CH Lin; methodology: CW Lin, TT Lai, SJ Chen, and CH Lin; resources: CW Lin, TT Lai, and CH Lin; supervision: CH Lin. validation: CW Lin and CH Lin; writing — original draft: CW Lin; writing — review and editing: CH Lin.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lin, CW., Lai, TT., Chen, SJ. et al. Elevated α-synuclein and NfL levels in tear fluids and decreased retinal microvascular densities in patients with Parkinson’s disease. GeroScience 44, 1551–1562 (2022). https://doi.org/10.1007/s11357-022-00576-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00576-6