Abstract
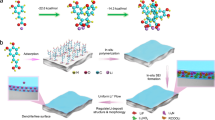
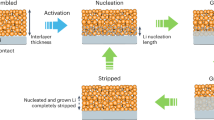
The low cycling efficiency and uncontrolled dendrite growth resulting from an unstable and heterogeneous lithium–electrolyte interface have largely hindered the practical application of lithium metal batteries. In this study, a robust all-organic interfacial protective layer has been developed to achieve a highly efficient and dendrite-free lithium metal anode by the rational integration of porous polymer-based molecular brushes (poly(oligo(ethylene glycol) methyl ether methacrylate)-grafted, hypercrosslinked poly(4‐chloromethylstyrene) nanospheres, denoted as xPCMS-g-PEGMA) with single-ion-conductive lithiated Nafion. The porous xPCMS inner cores with rigid hypercrosslinked skeletons substantially increase mechanical robustness and provide adequate channels for rapid ionic conduction, while the flexible PEGMA and lithiated Nafion polymers enable the formation of a structurally stable artificial protective layer with uniform Li+ diffusion and high Li+ transference number. With such artificial solid electrolyte interphases, ultralong-term stable cycling at an ultrahigh current density of 10 mA cm−2 for over 9,100 h (>1 year) and unprecedented reversible lithium plating/stripping (over 2,800 h) at a large areal capacity (10 mAh cm−2) have been achieved for lithium metal anodes. Moreover, the protected anodes also show excellent cell stability when paired with high-loading cathodes (~4 mAh cm−2), demonstrating great prospects for the practical application of lithium metal batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated in this study are included in the published article and its Supplementary Information.
Change history
06 May 2022
In the version of this article initially published, the scale bars shown in Fig. 2b,c and Fig. 6h were made proportionally incorrect during publisher processing, and have now been resized.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Cheng, X., Zhang, R., Zhao, C. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Dunn, B., Kamath, H. & Tarascon, J. Electrical energy storage for the grid: a battery of choices. Science 334, 928 (2011).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Zhang, X., Yang, Y. & Zhou, Z. Towards practical lithium-metal anodes. Chem. Soc. Rev. 49, 3040–3071 (2020).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Xin, S., Chang, Z., Zhang, X. & Guo, Y. Progress of rechargeable lithium metal batteries based on conversion reactions. Natl Sci. Rev. 4, 54–70 (2017).
Wang, H. et al. Alkali metal anodes for rechargeable batteries. Chem 5, 313–338 (2019).
Ma, J. et al. Prevention of dendrite growth and volume expansion to give high-performance aprotic bimetallic Li-Na alloy–O2 batteries. Nat. Chem. 11, 64–70 (2019).
Yan, C. et al. Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 30, 1707629 (2018).
Liu, Y. et al. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat. Energy 2, 17083 (2017).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Aurbach, D. et al. Recent studies of the lithium-liquid electrolyte interface electrochemical, morphological and spectral studies of a few important systems. J. Power Sources 54, 76–84 (1995).
Xu, R. et al. Artificial interphases for highly stable lithium metal anode. Matter 1, 317–344 (2019).
Yu, Z., Cui, Y. & Bao, Z. Design principles of artificial solid electrolyte interphases for lithium-metal anodes. Cell Rep. Phys. Sci. 1, 100119 (2020).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Kim, M. S. et al. Enabling reversible redox reactions in electrochemical cells using protected LiAl intermetallics as lithium metal anodes. Sci. Adv. 5, eaax5587 (2019).
Wang, Y. et al. Spherical Li deposited inside 3d Cu skeleton as anode with ultrastable performance. ACS Appl. Mater. Interfaces 10, 20244–20249 (2018).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 148, 405–416 (2002).
Bai, P. et al. Interactions between lithium growths and nanoporous ceramic separators. Joule 2, 2434–2449 (2018).
Stolz, L., Homann, G., Winter, M. & Kasnatscheew, J. Realizing poly(ethylene oxide) as a polymer for solid electrolytes in high voltage lithium batteries via simple modification of the cell setup. Mater. Adv. 2, 3251–3256 (2021).
Homann, G. et al. Poly(ethylene oxide)-based electrolyte for solid-state-lithium-batteries with high voltage positive electrodes: evaluating the role of electrolyte oxidation in rapid cell failure. Sci. Rep. 10, 4390 (2020).
Cheng, X. et al. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 3, 1500213 (2016).
Chen, H. et al. Uniform high ionic conducting lithium sulfide protection layer for stable lithium metal anode. Adv. Energy Mater. 9, 1900858 (2019).
Kozen, A. C. et al. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 9, 5884–5892 (2015).
Li, N., Yin, Y., Yang, C. & Guo, Y. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 28, 1853–1858 (2016).
Pathak, R. et al. Ultrathin bilayer of graphite/SiO2 as solid interface for reviving Li metal anode. Adv. Energy Mater. 9, 1901486 (2019).
Yan, C. et al. 4.5 V high-voltage rechargeable batteries enabled by the reduction of polarization on the lithium metal anode. Angew. Chem. Int. Ed. 58, 15235–15238 (2019).
Zhao, J. et al. Surface fluorination of reactive battery anode materials for enhanced stability. J. Am. Chem. Soc. 139, 11550–11558 (2017).
Li, N. et al. A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. Int. Ed. 57, 1505–1509 (2018).
Liu, K. et al. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 139, 4815–4820 (2017).
Sun, Y. et al. A novel organic “polyurea” thin film for ultralong-life lithium-metal anodes via molecular-layer deposition. Adv. Mater. 31, 1806541 (2019).
Wang, G. et al. Self-stabilized and strongly adhesive supramolecular polymer protective layer enables ultrahigh-rate and large-capacity lithium-metal anode. Angew. Chem. Int. Ed. 59, 2055–2060 (2020).
Zhu, B. et al. Poly(dimethylsiloxane) thin film as a stable interfacial layer for high-performance lithium-metal battery anodes. Adv. Mater. 29, 1603755 (2017).
Xu, R. et al. Dual-phase single-ion pathway interfaces for robust lithium metal in working batteries. Adv. Mater. 31, 1808392 (2019).
Xu, R. et al. Artificial soft–rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 28, 1705838 (2018).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Liu, X., Liu, J., Qian, T., Chen, H. & Yan, C. Novel organophosphate-derived dual-layered interface enabling air-stable and dendrite-free lithium metal anode. Adv. Mater. 32, 1902724 (2020).
Wu, C. et al. Mesoporous silica reinforced hybrid polymer artificial layer for high-energy and long-cycling lithium metal batteries. ACS Energy Lett. 5, 1644–1652 (2020).
Homann, G., Stolz, L., Winter, M. & Kasnatscheew, J. Elimination of “voltage noise” of poly (ethylene oxide)-based solid electrolytes in high-voltage lithium batteries: linear versus network polymers. iScience 23, 101225 (2020).
Zhao, Q. et al. Building organic/inorganic hybrid interphases for fast interfacial transport in rechargeable metal batteries. Angew. Chem. Int. Ed. 57, 992–996 (2018).
Kozen, A. C. et al. Stabilization of lithium metal anodes by hybrid artificial solid electrolyte interphase. Chem. Mater. 29, 6298–6307 (2017).
Liu, F. et al. Fabrication of hybrid silicate coatings by a simple vapor deposition method for lithium metal anodes. Adv. Energy Mater. 8, 1701744 (2018).
Pang, Q., Zhou, L. & Nazar, L. F. Elastic and Li-ion-percolating hybrid membrane stabilizes Li metal plating. Proc. Natl Acad. Sci. USA 115, 12389 (2018).
Balazs, A. C., Emrick, T. & Russell, T. P. Nanoparticle polymer composites: where two small worlds meet. Science 314, 1107 (2006).
Krishnamoorti, R. Strategies for dispersing nanoparticles in polymers. MRS Bull. 32, 341–347 (2007).
Xie, Y. et al. All-in-one porous polymer adsorbents with excellent environmental chemosensory responsivity, visual detectivity, superfast adsorption, and easy regeneration. Adv. Mater. 31, 1900104 (2019).
Mai, W. et al. Water-dispersible, responsive, and carbonizable hairy microporous polymeric nanospheres. J. Am. Chem. Soc. 137, 13256–13259 (2015).
Lutz, J., Lehn, J., Meijer, E. W. & Matyjaszewski, K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 1, 16024 (2016).
Zhou, M. et al. Ultrathin yet robust single lithium-ion conducting quasi-solid-state polymer-brush electrolytes enable ultralong-life and dendrite-free lithium-metal batteries. Adv. Mater. 33, 2100943 (2021).
Agapov, A. L., Wang, Y., Kunal, K., Robertson, C. G. & Sokolov, A. P. Effect of polar interactions on polymer dynamics. Macromolecules 45, 8430–8437 (2012).
Lian, H. et al. Enhanced actuation in functionalized carbon nanotube–Nafion composites. Sens. Actuators B 156, 187–193 (2011).
Martín, Z., Jiménez, I., Gómez-Fatou, M. A., West, M. & Hitchcock, A. P. Interfacial interactions in polypropylene–organoclay–elastomer nanocomposites: influence of polar modifications on the location of the clay. Macromolecules 44, 2179–2189 (2011).
Xu, Y. et al. Ion-transport-rectifying layer enables Li-metal batteries with high energy density. Matter 3, 1685–1700 (2020).
Meng, J., Chu, F., Hu, J. & Li, C. Liquid polydimethylsiloxane grafting to enable dendrite-free Li plating for highly reversible Li-metal batteries. Adv. Funct. Mater. 29, 1902220 (2019).
Tu, Z. et al. Fast ion transport at solid–solid interfaces in hybrid battery anodes. Nat. Energy 3, 310–316 (2018).
Kim, M. S. et al. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 3, 889–898 (2018).
Berg, E. J., Villevieille, C., Streich, D., Trabesinger, S. & Novák, P. Rechargeable batteries: grasping for the limits of chemistry. J. Electrochem. Soc. 162, A2468–A2475 (2015).
Cheng, X. et al. Dual-phase lithium metal anode containing a polysulfide-induced solid electrolyte interphase and nanostructured graphene framework for lithium–sulfur batteries. ACS Nano 9, 6373–6382 (2015).
Wu, J. et al. Polycationic polymer layer for air-stable and dendrite-free Li metal anodes in carbonate electrolytes. Adv. Mater. 33, 2007428 (2021).
Tang, W. et al. Lithium silicide surface enrichment: a solution to lithium metal battery. Adv. Mater. 30, 1801745 (2018).
Zhou, Y. et al. Redistributing Li-ion flux by parallelly aligned holey nanosheets for dendrite-free Li metal anodes. Adv. Mater. 32, 2003920 (2020).
Lee, D. et al. Copper nitride nanowires printed Li with stable cycling for Li metal batteries in carbonate electrolytes. Adv. Mater. 32, 1905573 (2020).
Cha, E. et al. 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li–S batteries. Nat. Nanotechnol. 13, 337–344 (2018).
Liang, X. et al. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2, 17119 (2017).
Pathak, R. et al. Fluorinated hybrid solid-electrolyte-interphase for dendrite-free lithium deposition. Nat. Commun. 11, 93 (2020).
He, G., Li, Q., Shen, Y. & Ding, Y. Flexible amalgam film enables stable lithium metal anodes with high capacities. Angew. Chem. Int. Ed. 58, 18466–18470 (2019).
Yan, C. et al. An armored mixed conductor interphase on a dendrite-free lithium-metal anode. Adv. Mater. 30, 1804461 (2018).
Adair, K. R. et al. Highly stable lithium metal anode interface via molecular layer deposition zircone coatings for long life next-generation battery systems. Angew. Chem. Int. Ed. 58, 15797–15802 (2019).
Yin, Y. et al. Metal chloride perovskite thin film based interfacial layer for shielding lithium metal from liquid electrolyte. Nat. Commun. 11, 1761 (2020).
Bai, M. et al. A scalable approach to dendrite-free lithium anodes via spontaneous reduction of spray-coated graphene oxide layers. Adv. Mater. 30, 1801213 (2018).
Salvatierra, R. V. et al. Suppressing Li metal dendrites through a solid Li-ion backup layer. Adv. Mater. 30, 1803869 (2018).
Guo, Y. et al. An autotransferable g-C3N4 Li+-modulating layer toward stable lithium anodes. Adv. Mater. 31, 1900342 (2019).
Shen, X. et al. Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery. Nat. Commun. 10, 900 (2019).
Gao, Y. et al. Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J. Am. Chem. Soc. 139, 15288–15291 (2017).
Zhang, X. et al. An extremely simple method for protecting lithium anodes in Li-O2 batteries. Angew. Chem. Int. Ed. 57, 12814–12818 (2018).
Zhang, K. et al. A high-performance lithium metal battery with ion-selective nanofluidic transport in a conjugated microporous polymer protective layer. Adv. Mater. 33, 2006323 (2021).
Gao, R. et al. Fatigue-resistant interfacial layer for safe lithium metal batteries. Angew. Chem. Int. Ed. 60, 25508–25513 (2021).
Gao, Y. et al. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019).
Jiang, Z. et al. Facile generation of polymer–alloy hybrid layers for dendrite-free lithium-metal anodes with improved moisture stability. Angew. Chem. Int. Ed. 58, 11374–11378 (2019).
Liu, S. et al. In situ solid electrolyte interphase from spray quenching on molten Li: a new way to construct high-performance lithium-metal anodes. Adv. Mater. 31, 1806470 (2019).
Gu, Y. et al. Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat. Commun. 9, 1339 (2018).
Lu, D. et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015).
Niu, C. et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 6, 723–732 (2021).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51925308 and 51872336), the National Key Research and Development Program of China (2021YFF0500600), the Leading Scientific, Technical and Innovation Talents of the Guangdong Special Support Program (2017TX04C248), the Pearl River Talent Plan of Guangdong (2017GC010612), the Natural Science Foundation of Guangdong (2021A1515011617), the Fundamental Research Funds for the Central Universities (20lgzd18) and the Science and Technology Program of Guangzhou (202102021111 and 202002020041).
Author information
Authors and Affiliations
Contributions
D.W. conceived the concept and supervised the research. S. Liu and D.W. designed the project. S. Li synthesized the materials. S. Li, J.H., Y.C., Z.C., W.H. and C.L. performed the material characterization and electrochemical tests. S. Li, S. Liu and D.W. wrote the manuscript. R.L. and R.F. gave advice on the research. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Qiang Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31 and Table 1.
Supplementary Video 1
In operando observation of Li deposition on bare Li.
Supplementary Video 2
In operando observation of Li deposition on the xPCMS-g-PEGMA/LN@Li anode.
Rights and permissions
About this article
Cite this article
Li, S., Huang, J., Cui, Y. et al. A robust all-organic protective layer towards ultrahigh-rate and large-capacity Li metal anodes. Nat. Nanotechnol. 17, 613–621 (2022). https://doi.org/10.1038/s41565-022-01107-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01107-2
This article is cited by
-
External-pressure–electrochemistry coupling in solid-state lithium metal batteries
Nature Reviews Materials (2024)
-
Operando monitoring of dendrite formation in lithium metal batteries via ultrasensitive tilted fiber Bragg grating sensors
Light: Science & Applications (2024)
-
Towards Routine 7Li In Situ Solid-State NMR Studies of Electrochemical Processes in Li|LiPF6|LFP Cells
Applied Magnetic Resonance (2024)
-
Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries
Nature Energy (2023)
-
Production of high-energy 6-Ah-level Li | |LiNi0.83Co0.11Mn0.06O2 multi-layer pouch cells via negative electrode protective layer coating strategy
Nature Communications (2023)