Abstract
Arrhythmogenic cardiomyopathy (AC) is an inherited disorder characterized by lethal arrhythmias and a risk to sudden cardiac death. A hallmark feature of AC is the progressive replacement of the ventricular myocardium with fibro-fatty tissue, which can act as an arrhythmogenic substrate further exacerbating cardiac dysfunction. Therefore, identifying the processes underlying this pathological remodelling would help understand AC pathogenesis and support the development of novel therapies. In this review, we summarize our knowledge on the different models designed to identify the cellular origin and molecular pathways underlying cardiac fibroblast and adipocyte cell differentiation in AC patients. We further outline future perspectives and how targeting the fibro-fatty remodelling process can contribute to novel AC therapeutics.
Similar content being viewed by others
Introduction
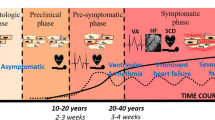
Arrhythmogenic cardiomyopathy (AC) is a genetic disorder that usually manifests with three main clinical phases: (I) the concealed phase, with minimal clinical symptoms, but with a risk of sudden cardiac death; (II) the electrical phase, with symptomatic ventricular arrhythmias in the form of ventricular tachycardias or ventricular fibrillation, palpitations, and syncope, together with structural abnormalities; and (III) the end-stage phase, with severe structural changes, ventricular dilation, and dysfunction which can progress to heart failure [4, 16, 58]. The prevalence of AC is 1:2000 to 1:5000 depending on the population, and has been found to mainly affect young individuals and athletes [17, 77]. Despite the complex disease aetiology, with multiple risk factors contributing to disease pathogenesis, genetic predisposition remains the main underlying cause of AC. A familial background has been associated with about 60% of AC cases with an autosomal dominant mode of inheritance [36, 45]. Most of AC-related mutations (~ 54% of all AC cases) have been linked to genes encoding components of desmosomes; intercellular junctions expressed by cardiac muscle and epithelial tissue. These include plakophilin-2 (PKP2), desmoplakin (DSP), desmocollin-2 (DSC2), desmoglein-2 (DSG2) and plakoglobin (JUP). However, mutations in the non-desmosomal genes transmembrane protein 43 (TMEM43), phospholamban (PLN), transforming growth factor beta 3 (TGFB3), desmin (DES), titin (TTN), αT-catenin (CTNNA3), and lamin A/C (LMNA), have been also linked to AC in ~ 5% of the cases [36, 45].
The most striking histopathological feature of AC is the loss of ventricular myocardium, possibly due to cardiomyocyte atrophy and apoptosis, and its replacement with fibro-fatty tissue. Fibro-fatty tissue deposition typically extends from the epicardium towards the endocardium and is usually associated with inflammatory infiltrates [5, 16] (Fig. 1). As the disease progresses, fibro-fatty tissue can act as a substrate to aggravate arrhythmias further hindering proper ventricular function [20, 76].
Cardiac fibro-fatty tissue remodelling in AC patients. Masson trichrome staining of explanted human hearts from a healthy control and AC patients showing cardiomyocytes in red, fibrosis in blue and adipocytes in white. Upper panels show full ventricular sections and insets indicate slices where higher magnification images were obtained. Lower panels show fibro-fatty tissue replacement within myocardial regions of the corresponding ventricular slices. RV, right ventricle; LV, left ventricle.
Many cellular and animal models of AC are available to help understand different aspects of AC pathogenesis such as ventricular dysfunction, cardiomyocyte death and inflammation [3, 33]. However, recapitulating and studying the fibro-fatty infiltration process characteristic of AC has been limited due to the natural resistance of most experimental animal models to cardiac adipose tissue development [82]. In this review, we summarize our understanding on the cellular origin and mechanisms of fibroblast and fat cell differentiation in AC.
Cellular origin
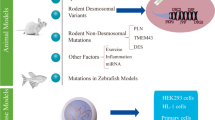
In recent years, there has been considerable efforts to elucidate the cellular effectors contributing to the replacement of ventricular myocardial tissue with extensive fibro-fatty infiltrates in AC patients. Studies implemented different models of dysfunctional desmosomes due to mutations or gene knockouts. These included mouse and zebrafish as well as cellular models of AC such as human-induced pluripotent stem cell (hiPSC)-derived cardiac cultures and explanted human AC hearts. So far, cell types which have been proposed to act as a source of fibro-fatty tissue in AC are cardiomyocytes, cardiac progenitor cells and epicardial cells. These studies are summarized in Table 1 and discussed below.
Cardiomyocytes
Cardiomyocyte transdifferentiation into adipocytes during AC progression was first suggested based on the morphological examination of the ventricular myocardium of a female transplant patient [18]. Cardiomyocytes adjacent to fibro-fatty tissue contained sarcoplasmic vacuoles with a lipidic nature that highly resembled pre-adipocytes [18]. The authors therefore suggested a cardiomyocyte-to-adipocyte switch, as some of these cells also stained positive for vimentin, a marker expressed by adipocytes [18]. However, vimentin is a mesenchymal marker which is not exclusively expressed by adipocytes [19]. The presence of intracellular lipid droplets in cardiomyocytes has been also described in biopsied myocardial tissue of another AC patient [30]. These lipids were found in degenerating cardiomyocytes and were often discharged into the interstitial space upon cell membrane dissociation [30]. Despite the informative ultrastructural examination of explanted AC hearts, these studies were based on the histological observation of single cases.
In an in vivo setting, one report could show that some adipocytes arising in a Dsp knockout mouse model located at the sub-epicardium, but not at the midwall, originate from a cardiomyocyte lineage labelled by MLC2v [55]. Presence of a common “cardiomyocyte-adipocyte progenitor” cell population in normal hearts was further proposed in a study by Dorn et al. [24]. The authors described an Isl1+/Wt1+ progenitor cell population in normal hearts, which under different stimuli primes towards a myocytic or adipocytic fate, and hence could potentially contribute to adipocyte differentiation in AC [24].
Due to difficulties with recapitulating the fibro-fatty phenomenon in many AC mouse models, the use of hiPSCs presented an alternative tool to study the cardiomyocyte transdifferentiation hypothesis in vitro. In three subsequent reports, hiPSC-derived cardiomyocytes (hiPSC-CMs) generated from AC patients demonstrated several AC features such as reduced densities of desmosomal proteins and electrical instabilities [10, 42, 56]. Additionally, exposure of hiPSC-CMs to adipogenic stimuli induced lipid droplet accumulation, which suggested an underlying predisposition to adipocytic differentiation in AC [10, 42, 56]. However, the exposure of cells to an adipogenic environment is rather artificial and does not mimic human disease, hence the cardiomyocyte-to-adipocyte transdifferentiation theory requires further investigation.
Cardiac progenitor cells
The presence of unique progenitor cell populations in the heart with a multipotent differentiation potential has gained much attention in the recent years. Different reports have suggested that during AC disease progression, resident cardiac progenitors can differentiate into fibroblasts, adipocytes, or both. Below, we discuss the different populations proposed.
Isl1+ Mef2c+ progenitors
In 2009, the group of A. J. Marian used lineage tracing to monitor the origin of adipocytes in Dsp-deficient mice [54]. Three cardiac lineage promoters were used: Nkx2.5 which labels descendants of first and second heart field progenitors as well as epicardial cells, Mef2c which labels descendants of only second heart field progenitors, and Myh6 which labels cardiomyocytes [54]. Most adipocytes in the Dsp-deficient mice were shown to derive from the Nkx2.5 and Mef2c lineage, but not from the Myh6 lineage, indicating that adipocytes in AC hearts can possibly arise from second heart field progenitors expressing Isl1 and Mef2c [54]. This has been suggested to occur due to PKG translocation to the nucleus which leads to suppressed WNT signalling and hence enhanced adipogenic differentiation in AC hearts [54]. This study demonstrates the possible contribution of second heart field progenitors to adipocytes arising in the right ventricle. However, presence of biventricular and left-dominant forms of AC argues against a second heart field origin of adipocytes.
c-Kit+ Sca1+ progenitors
To follow up on the role of PKG nuclear translocation and its role in AC pathogenesis, the same group further investigated the relation between PKG, WNT signalling and adipogenesis [53]. The authors generated transgenic mice overexpressing wild-type (PKGWT) or truncated PKG (PKGTR) as well as PKG null mice (PKG−/−) [53]. PKGTR mice showed reduced membrane localization and binding to DSP and DSG2 [53]. Furthermore, PKG was found to be expressed in a progenitor cell population expressing c-Kit and Sca1, which, upon adipogenic stimulation, could undergo lipogenesis in vitro [53]. This effect was shown to be mediated through suppressed WNT signalling and reversed through WNT signalling activation [53]. Furthermore, c-Kit+ Sca1+ progenitors isolated from PKG−/− embryos were resistant to adipogenesis and exhibited increased levels of WNT signalling activation, further suggesting the potential of c-Kit+ Sca1+ progenitors to undergo adipogenic differentiation [53]. However, the extremely low abundance of c-Kit+ Sca1+ cells in the heart, and emerging reports arguing against their pluripotent potential [75], limit their ability to act as a main source of adipocytes in AC.
Fibro-adipocyte progenitors
Fibro-adipocyte progenitors (FAPs) were first described in skeletal muscle as a quiescent population of cells which can rapidly proliferate and contribute to adipocyte and fibroblast differentiation after muscle injury [40, 78]. In the heart, Marian’s group could further identify a similar PDGFRA+ cell population with bipotential towards fibroblast and adipocyte differentiation [52]. Isolation of FAPs from human and mouse hearts showed that these cells expressed COL1A1 or CEBPA, which allowed to label them as fibroblast or adipocyte progenitors, respectively [52]. In a Dsp-deficient mouse model, the authors could show that ~ 40% of cardiac adipocytes originated from FAPs, indicating the contribution of FAPs, as well as other cell types, to adipogenesis in AC [52]. Corroborating their previous AC studies, the adipogenic potential of FAPs was found to be mediated through WNT signalling suppression, which when re-activated reduced adipogenesis in vitro [52]. Although the described FAPs population was selected based on the exclusion of the fibroblast markers THY1 and DDR2, most of these cells still expressed COL1A1, which is often depicted as an activated myofibroblast marker. Furthermore, interestingly, DSP was only expressed in the adipogenic, and not fibrogenic, subsets of FAPs, which made it difficult to trace the fibrogenic potential of Dsp-deficient FAPs [52].
Later in 2020, another study further described the role FAPs in models of myocardial infarction (MI) and AC [71]. The authors described FAPs as a multipotent resident progenitor cell population expressing PDGFRA and the progenitor cell markers SCA1 and HIC1 [71]. In response to MI, these cells were activated generating fibroblasts which contributed to scar tissue formation at the injury area [71]. In an AC mouse model overexpressing mutant DSG2, cells derived from the PDGFRA+ or HIC1+ lineage contributed to both fibroblast and adipocyte differentiation [71]. A similar phenotype was observed in Hic1 knockout hearts [71]. However, in this study, it was unclear how different injury models triggered fibrogenesis only or fibro-adipogenesis following HIC1 suppression.
Mesenchymal stromal cells
Cardiac mesenchymal stem/stromal cells (MSCs) are multipotent progenitors of epicardial origin, which are important for the mechanical support of tissues by providing extracellular matrix and paracrine signals [63]. The contribution of cardiac resident MSCs to scar tissue formation was first reported in a model of MI [9]. The authors could show that CD44+ MSCs act as precursor cells which generate scar tissue fibroblasts upon injury [9]. In vitro, these cells expressed both stem cell and fibroblast markers and retained a self-renewal and multipotent capability when cultured in different lineage-induction media [9].
In the context of AC, a study by Sommariva et al. showed their potential to also differentiate into adipocytes [72]. In hearts of AC patients, adipocytes labelled by the mature adipocyte marker PLIN1 also stained positive for the mesenchymal markers CD29 and CD105, suggesting their mesenchymal origin [72]. Of note, the authors occasionally found rare populations of c-Kit+ adipocytes suggesting a possible, but limited, contribution of c-Kit+ progenitors to adipogenesis [72]. MSCs isolated from AC patients expressed higher levels of adipogenic markers and were more prone to undergo lipogenesis than control MSCs when subjected to adipogenic stimuli [72]. This effect was reduced following WNT pathway activation or PKP2 overexpression in patient MSCs [72]. Due to their high abundance in the heart and plasticity to differentiate into various mesenchymal lineages, MSCs present strong candidates to adipogenesis in AC. However, the isolation of these cells relies on their plastic adherence after digestion and their expression of mesenchymal markers (CD90, CD29, CD105, CD44, CD73) [66]. These properties are not specific for MSCs, but also found in other stromal cells such as fibroblasts [28, 66]. Furthermore, MSCs were isolated from patient auricles and not ventricles which show most fibro-fatty remodelling. In addition to their reported adipogenic potential in this study, it would be interesting to investigate the fibrogenic potential of these MSCs as well, owing to their previously described role in scar tissue formation following ischemic injury [9].
Epicardial cells
The epicardium is the outermost layer of the heart composed of mesothelial cells that mainly remain quiescent in adult hearts. During development and after disease, epicardial cells can undergo epithelial-to-mesenchymal transition (EMT) giving rise to different cardiac cell populations [34, 70]. Given their multipotent cell potential [34, 70], the sub-epicardial predominance of fibro-fatty infiltrates in AC patients [45, 76], and the high epicardial expression of desmosomal genes [43, 60], epicardial cells have been also suggested as candidates to originate fibro-fatty tissue.
In the normal murine heart, fat tissue is often confined at a specific region named the atrial–ventricular (AV) groove [82]. A study by Yamaguchi et al. demonstrated that the AV groove fat originates from the epicardium through the EMT and PPARγ pathway activation [82]. Later, using lineage tracing on ischemic injury models, adipocytes emerging at the peri-infarct region following MI were shown to also partially derive from the adult epicardium [51, 86]. Additionally, epicardial-to-fibroblast differentiation due to adult epicardial EMT has been also suggested in models of MI [26, 68, 80].
Interestingly, in studies on atrial fibrillation, which is characterized by the extensive atrial remodelling with fibro-fatty tissue, fibroblasts and adipocytes were shown to arise from the epicardium [74]. This appeared to be due to a pre-programmed state of subsets of adult epicardial-derived cells (EPDCs) towards either fibroblast or adipocyte cell fates, which when activated undergo fibro-fatty differentiation and infiltration into the diseased atria [74].
In the context of AC, Matthes et al. were the first to show that epicardial explants from neonatal rat hearts express PKP2, which when silenced promotes cellular proliferation, migration, lipogenesis and cellular differentiation into α-SMA+ cells [60]. These data suggested an important role of the desmosome in maintaining the mechanical integrity of epicardial cells, which when lost could potentially promote cellular differentiation.
In addition to the previously discussed studies from Marian’s group suggesting the contribution of different progenitors to fibro-fatty differentiation [52,53,54], they could recently also demonstrate the role of the epicardium in AC pathogenesis [85]. Using a reporter mouse model carrying an inducible epicardial-specific Dsp deletion, the authors could show the epicardial origin of fibroblasts [85]. These cells were shown to express paracrine factors such as TGFβ1 and FGF, which mediate EMT as well as apoptosis, arrhythmias and cardiac dysfunction [85].
In a recently published report, our group further underscored the role of the epicardium in fibro-fatty remodelling. [43]. In this study, we made use of hiPSC-derived cardiac cultures generated from AC patients, their isogenic controls and healthy donors to study AC pathogenesis in vitro. We could show that hiPSC-derived epicardial cells undergo EMT and spontaneous fibro-fatty cellular differentiation upon desmosomal gene suppression, due to either intrinsic mutations or targeting siRNAs. Using single cell RNA sequencing, we identified transcription factor TFAP2A to mediate this process by enhancing EMT signalling in the diseased cells. Furthermore, we observed that human AC hearts display increased epicardial thickening and WT1 expression indicative of epicardial activation. Additionally, cells located at the sub-epicardial mesenchyme stained positive for WT1 and TFAP2A, which further suggested that an epicardial-derived subset of cells underlies the fibro-fatty phenotype.
Other potential sources of fibroblasts
Various studies have tried elucidating the cellular origin of fibroblasts contributing to scar tissue formation in different forms of cardiovascular disease. Although not particularly studied in AC, these identified fibroblast progenitor populations might also potentially contribute to fibrosis in AC patients and hence are also discussed below.
All resident fibroblasts in the adult heart derive from the epicardium during development and remain quiescent under homeostatic conditions [31]. Several reports could demonstrate the presence of cardiac fibroblast progenitor cell populations which upon injury can contribute to the pathological fibroblast differentiation. In one study, an adult stem cell population labelled by PW1 was found to contribute to fibrotic remodelling after MI [83]. PW1+ cells were mainly found near infarct areas of human and mouse ischemic hearts and could give rise to fibroblasts labelled by FSP1 and α-SMA [83]. In another study, peri-vascular precursors, labelled by GLI1 expression, were also shown to contribute to fibrosis after injury in different organs including the heart [44]. Lineage tracing experiments showed that 60% of infarct fibroblasts arise from GLI1 + vascular progenitors [44].
Bone marrow-derived cells have been also suggested to differentiate into cardiac fibroblasts, although the contribution seemed to vary between different cardiac disorders. In different studies, mice were transplanted with genetically labelled or sex-mismatched bone marrow cells which were shown to significantly contribute to scar tissue fibroblasts after MI [61, 79] and in dilated cardiomyopathy [15].
Another study described a circulating population of leukocytes termed fibrocytes as a source of injury fibroblasts in models of angiotensin-II-induced cardiac hypertrophy [38] and ischemic cardiomyopathy [39]. These cells normally express both hematopoietic markers (CD34, CD45) and fibroblast markers (procollagen I, vimentin) [48]. Upon stimulation, fibrocytes are recruited to the injury site where they adopt a myofibroblast phenotype expressing α-SMA and produce collagen [48].
In contrast to these reports, other lineage-tracing studies could show that scar tissue fibroblasts mainly originate from pre-existing resident epicardium-derived fibroblasts and not from bone marrow or stem cell populations. This was observed in models of pressure overload [1, 62] and MI [29, 41, 67, 84].
Despite the potential of the described reports to identify the origin of fibroblasts in different cardiac disorders, it is unknown whether these findings also apply to AC where fibroblasts infiltrate massive regions of adipose tissue.
Pathways implicated
AC is mainly considered a disease of cardiac desmosomes [21]. These multiprotein complexes are required for maintaining both mechanical and electrical signals throughout the heart [21]. The main pathways found to be potentially implicated in fibro-fatty remodelling in AC due to desmosomal dysregulation are WNT, Hippo, TGFβ and PPARγ signalling pathways.
WNT pathway
Canonical WNT signalling is the most commonly accepted pathway involved in AC pathogenesis. β-catenin, together with PKG, also known as γ-catenin, acts to link cadherin proteins to the actin cytoskeleton [88]. In response to WNT ligands, intracellular β-catenin levels are stabilized leading to its translocation to the nucleus, where it activates target genes via binding to TCF/LEF1 transcription factors [88]. Since PKG functions as a constituent of the desmosome and has been also shown to inhibit the transcriptional activity of β-catenin [64, 88], WNT signalling has been suggested to be implicated in AC pathogenesis.
Aberrant WNT signalling in AC was first reported by Garcia-Gras et al. in 2006 [32]. The authors showed that loss of DSP expression in cultured atrial myocytes or in mice leads to the nuclear translocation of PKG, where it competes with β-catenin for binding to TCF/LEF1 and therefore inhibits β-catenin-mediated WNT signalling [32]. Since WNT signalling inhibition is known to promote adipogenesis [65], the authors suggested PKG nuclear translocation as an underlying mechanism to adipocyte differentiation in AC [32]. Reduced WNT signalling activity has been further demonstrated in other AC mouse models overexpressing mutant forms of Jup [53] and Dsg2 [8] and in Dsp-deficient zebrafish [35]. However, conflicting to these data, cardio-restricted Jup knockout was shown to increase β-catenin stabilization and TCF/LEF transcriptional activity, which was suggested to underlie a cardiac hypertrophic phenotype in these mice with no signs of cardiac adipogenesis [47]. In another cardiac-specific Jup knockout mouse model, WNT signalling was shown to be unaltered [46]. In addition to its involvement in adipogenic remodelling, WNT signalling has been also linked to cardiomyocyte apoptosis and electrical instabilities [2, 37], suggesting a broader role for WNT signalling in AC pathogenesis. However, further investigation is needed to understand the causal mechanisms underlying WNT pathway dysregulation in AC patients.
Hippo pathway
The Hippo pathway is well known for its roles in regulating cellular proliferation and apoptosis, thereby controlling growth of tissues [81]. Particularly in AC, a study showed that in human AC hearts, as well as in cell culture and mouse models of AC, Hippo signalling was activated [13]. This led to the phosphorylation of downstream Hippo molecules including the Hippo-effector molecule Yes-associated protein (YAP), which was found to interact with β-catenin and PKG, ultimately enhancing the adipogenic phenotype [13].
TGFβ pathway
TGFβ signalling is key in regulating cardiac fibrosis and hence has been linked with fibrotic remodelling in AC [23, 49]. The association of TGFβ signalling with AC was first demonstrated by the identification of mutations in the UTR regions of TGFβ3 in two familial cases of AC, which were shown to increase the activity of TGFβ by twofold [7]. Furthermore, in a Jup knockout mouse model, which showed massive fibrosis and no alterations in WNT signalling, TGFβ signalling was significantly induced [46]. The authors suggested that the elevated TGFβ activity could be contributed to an increase in myocardial wall stress as a result of desmosomal loss [46]. Interestingly, the increase in TGFβ activity has recently also been validated in plasma collected from AC patients, which coincided with an increase in fibrotic markers in endomyocardial biopsies [57]. In this study, the authors found that MSCs isolated from AC patients were more prone to fibrotic differentiation in response to TGFβ1 treatment compared to healthy donors [57]. In another study, knockdown of Pkp2 in cardiomyocytes induced a TGFβ-mediated induction of pro-fibrotic genes [27]. This effect was corroborated in tissues isolated from Pkp2 and Dsp-deficient mouse and zebrafish models [27, 35]. In addition to its role in fibrotic differentiation, TGFβ is known for its crucial roles in promoting EMT signalling in the heart which can precede fibrotic remodelling [6, 25]. Recently, we could show that hiPSC-epicardial cells and primary human atrial EPDCs undergo excessive EMT in response to TGFβ1 treatment or desmosomal suppression [43]. This has been further corroborated in an epicardial-specific Dsp knockout mouse model which demonstrated an epicardial-derived origin of fibroblasts due to enhanced EMT and TGFβ signalling [85]. However, the exact link between AC-related mutations and EMT remains to be elucidated.
PPARγ pathway
PPARγ is a nuclear receptor that functions as a master regulator of lipid uptake and adipogenesis [11]. Induction of PPARγ signalling together with increased lipogenesis have been described in different models of AC. These include Dsp- and Pkp2-deficient cardiomyocytes [24, 32, 42] and epicardial cells [43] as well as human AC hearts [22]. The exact mechanisms by which desmosomal dysregulation alters PPARγ signalling are not fully understood. However, one possible link could be the negative regulation of PPARγ by β-catenin [50], which is widely suggested to be inhibited in AC [8, 32, 35, 53].
Concluding remarks
AC is a multifaceted and progressive disorder. Its pathogenesis usually initially manifests with electrophysiological instabilities which can lead to lethal ventricular arrhythmias [4]. As the disease develops, fibro-fatty remodelling progressively intervenes, which can act as an arrhythmogenic substrate further hindering cardiac conductivity [20, 76]. In this review, we discussed the possible cellular origins and mechanisms which can underlie fibro-fatty tissue deposition in AC hearts (Fig. 2). However, whether the emergence of fibro-fatty tissue is a direct cause of AC-related mutations, a consequence to cardiomyocyte death and physiological instabilities, or possibly both, remains an open question.
Schematic of the different cellular populations and pathways reported to underlie fibro-fatty remodelling in AC. Fibro-fatty tissue typically extends from the epicardium towards the myocardium in AC hearts. Depicted arrows indicate the potential of each cell population to differentiate into another and the proposed pathways or processes underlying this transition. AC arrhythmogenic cardiomyopathy; Wnt Wingless-related integration site; Pparγ peroxisome proliferator–activated receptor gamma; Tgfβ transforming growth factor beta; EMT epithelial-to-mesenchymal transition
As previously outlined, cardiomyocyte-to-fibroblast/adipocyte transdifferentiation is potentially limited in AC hearts. However, emerging evidence suggests the presence of resident cardiac cell populations with ability to differentiate into fibroblasts, adipocytes or both. It is important to note that not a single cell population should be considered the only source to cardiac fibro-adiposis, as many populations can possibly overlap at different developmental and pathological stages. As discussed, the epicardium presents a strong candidate to fibro-fatty cellular differentiation. However, Isl1+ progenitors, which were suggested to originate adipocytes in AC, can also give rise to epicardium [87]. Additionally, FAPs and MSCs, which seem to present overlapping cell populations, can be derived via epicardial EMT [14, 72], and hence can act as intermediate multipotent mesenchymal cell populations (Fig. 2).
From a clinical perspective, current AC treatments are mainly directed towards relieving symptoms and halting disease progression to heart failure. These include antiarrhythmic drugs, implantable cardioverter defibrillators, catheter ablation, and optimally heart transplantation [17]. However, with the ongoing molecular understanding to AC pathogenesis, treatments targeted towards specific molecular pathways present promising therapeutic alternatives. Using a high-throughput screening approach, the WNT pathway activator SB216763 was identified as a novel candidate to prevent cardiac dysfunction in zebrafish and mouse models of AC [2, 12]. However, specifically related to fibro-fatty remodelling, difficulties with recapitulating this phenomenon in murine AC models has halted testing potential targeted therapies in a pre-clinical setting. However, one recent study showed that boosting levels of oxidized low-density lipoprotein (oxLDL) through high fat diet feeding in Pkp2 heterozygous knockout mice that normally display no overt phenotype leads to sub-epicardial adipogenesis and ventricular systolic impairment [73]. Since AC patients with severe cardiac dysfunction and fibro-fatty tissue display high plasma levels of oxLDL, these mice could serve as suitable models to test the potential of targeting fibro-fatty tissue in vivo [73]. Additionally, advances in hiPSC technologies have allowed mimicking human AC pathogenesis and studying key pathological pathways of the disease in vitro. By generating patient-specific cardiac cultures, our group recently showed that siRNA-mediated epicardial targeting of transcription factor TFAP2A can reduce AC-induced EMT and fibro-fatty differentiation, which remains to be validated in a pre-clinical setting [43].
Owing to the sub-epicardial pre-dominance of fibro-fatty infiltrates in AC patients, therapies can potentially be conveniently directed towards the pericardial sac or the epicardial membrane using patches, catheters and slow-release hydrogels [59]. However, careful assessment to off-target effects, identifying optimal delivery systems, and whether a preventative therapy can be used at early stages of the disease, when fibro-fatty remodelling has not yet outspread in the heart, are yet to be studied. This will potentially help design better therapeutic options for patients with AC as well as other forms of cardiac disease.
References
Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R (2014) Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 115:625–635. https://doi.org/10.1161/CIRCRESAHA.115.303794
Asimaki A, Kapoor S, Plovie E, Karin AA, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kleber AG, Saffitz JE (2014) Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med 6:240ra274. https://doi.org/10.1126/scitranslmed.3008008
Austin KM, Trembley MA, Chandler SF, Sanders SP, Saffitz JE, Abrams DJ, Pu WT (2019) Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat Rev Cardiol 16:519–537. https://doi.org/10.1038/s41569-019-0200-7
Basso C, Corrado D, Bauce B, Thiene G (2012) Arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 5:1233–1246. https://doi.org/10.1161/CIRCEP.111.962035
Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M (1996) Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 94:983–991. https://doi.org/10.1161/01.cir.94.5.983
Bax NA, van Oorschot AA, Maas S, Braun J, van Tuyn J, de Vries AA, Groot AC, Goumans MJ (2011) In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFbeta-signaling and WT1. Basic Res Cardiol 106:829–847. https://doi.org/10.1007/s00395-011-0181-0
Beffagna G, Occhi G, Nava A, Vitiello L, Ditadi A, Basso C, Bauce B, Carraro G, Thiene G, Towbin JA, Danieli GA, Rampazzo A (2005) Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res 65:366–373. https://doi.org/10.1016/j.cardiores.2004.10.005
Calore M, Lorenzon A, Vitiello L, Poloni G, Khan MAF, Beffagna G, Dazzo E, Sacchetto C, Polishchuk R, Sabatelli P, Doliana R, Carnevale D, Lembo G, Bonaldo P, De Windt L, Braghetta P, Rampazzo A (2019) A novel murine model for arrhythmogenic cardiomyopathy points to a pathogenic role of Wnt signalling and miRNA dysregulation. Cardiovasc Res 115:739–751. https://doi.org/10.1093/cvr/cvy253
Carlson S, Trial J, Soeller C, Entman ML (2011) Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc Res 91:99–107. https://doi.org/10.1093/cvr/cvr061
Caspi O, Huber I, Gepstein A, Arbel G, Maizels L, Boulos M, Gepstein L (2013) Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ Cardiovasc Genet 6:557–568. https://doi.org/10.1161/CIRCGENETICS.113.000188
Chandra M, Miriyala S, Panchatcharam M (2017) PPARgamma and Its Role in Cardiovascular Diseases. PPAR Res 2017:6404638. https://doi.org/10.1155/2017/6404638
Chelko SP, Asimaki A, Andersen P, Bedja D, Amat-Alarcon N, DeMazumder D, Jasti R, MacRae CA, Leber R, Kleber AG, Saffitz JE, Judge DP (2016) Central role for GSK3beta in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight. https://doi.org/10.1172/jci.insight.85923
Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ (2014) The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res 114:454–468. https://doi.org/10.1161/CIRCRESAHA.114.302810
Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A, Biben C, Zoellner H, Colvin EK, Pimanda JE, Biankin AV, Zhou B, Pu WT, Prall OW, Harvey RP (2011) Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 9:527–540. https://doi.org/10.1016/j.stem.2011.10.002
Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, Kaye DM (2010) Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol 176:1735–1742. https://doi.org/10.2353/ajpath.2010.090574
Corrado D, Link MS, Calkins H (2017) Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 376:61–72. https://doi.org/10.1056/NEJMra1509267
Corrado D, Thiene G (2006) Arrhythmogenic right ventricular cardiomyopathy/dysplasia: clinical impact of molecular genetic studies. Circulation 113:1634–1637. https://doi.org/10.1161/CIRCULATIONAHA.105.616490
d’Amati G, di Gioia CR, Giordano C, Gallo P (2000) Myocyte transdifferentiation: a possible pathogenetic mechanism for arrhythmogenic right ventricular cardiomyopathy. Arch Pathol Lab Med 124:287–290. https://doi.org/10.5858/2000-124-0287-MT
Danielsson F, Peterson MK, Caldeira Araujo H, Lautenschlager F, Gad AKB (2018) Vimentin Diversity in Health and Disease. Cells. https://doi.org/10.3390/cells7100147
De Coster T, Claus P, Kazbanov IV, Haemers P, Willems R, Sipido KR, Panfilov AV (2018) Arrhythmogenicity of fibro-fatty infiltrations. Sci Rep 8:2050. https://doi.org/10.1038/s41598-018-20450-w
Delmar M, McKenna WJ (2010) The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res 107:700–714. https://doi.org/10.1161/CIRCRESAHA.110.223412
Djouadi F, Lecarpentier Y, Hebert JL, Charron P, Bastin J, Coirault C (2009) A potential link between peroxisome proliferator-activated receptor signalling and the pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Res 84:83–90. https://doi.org/10.1093/cvr/cvp183
Dobaczewski M, Chen W, Frangogiannis NG (2011) Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 51:600–606. https://doi.org/10.1016/j.yjmcc.2010.10.033
Dorn T, Kornherr J, Parrotta EI, Zawada D, Ayetey H, Santamaria G, Iop L, Mastantuono E, Sinnecker D, Goedel A, Dirschinger RJ, My I, Laue S, Bozoglu T, Baarlink C, Ziegler T, Graf E, Hinkel R, Cuda G, Kaab S, Grace AA, Grosse R, Kupatt C, Meitinger T, Smith AG, Laugwitz KL, Moretti A (2018) Interplay of cell-cell contacts and RhoA/MRTF-A signaling regulates cardiomyocyte identity. EMBO J. https://doi.org/10.15252/embj.201798133
Dronkers E, Wauters MMM, Goumans MJ, Smits AM (2020) Epicardial TGFbeta and BMP signaling in cardiac regeneration: what lesson can we learn from the developing heart? Biomolecules. https://doi.org/10.3390/biom10030404
Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A, Majesky M, Deb A (2012) Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J 31:429–442. https://doi.org/10.1038/emboj.2011.418
Dubash AD, Kam CY, Aguado BA, Patel DM, Delmar M, Shea LD, Green KJ (2016) Plakophilin-2 loss promotes TGF-beta1/p38 MAPK-dependent fibrotic gene expression in cardiomyocytes. J Cell Biol 212:425–438. https://doi.org/10.1083/jcb.201507018
Floy ME, Mateyka TD, Foreman KL, Palecek SP (2020) Human pluripotent stem cell-derived cardiac stromal cells and their applications in regenerative medicine. Stem Cell Res 45:101831. https://doi.org/10.1016/j.scr.2020.101831
Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, Molkentin JD (2018) Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest 128:2127–2143. https://doi.org/10.1172/JCI98215
Fujita S, Terasaki F, Otsuka K, Katashima T, Kanzaki Y, Kawamura K, Tanaka T, Kitaura Y (2008) Markedly increased intracellular lipid droplets and disruption of intercellular junctions in biopsied myocardium from a patient with arrhythmogenic right ventricular cardiomyopathy. Heart Vessels 23:440–444. https://doi.org/10.1007/s00380-008-1079-0
Furtado MB, Costa MW, Rosenthal NA (2016) The cardiac fibroblast: Origin, identity and role in homeostasis and disease. Differentiation 92:93–101. https://doi.org/10.1016/j.diff.2016.06.004
Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ (2006) Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest 116:2012–2021. https://doi.org/10.1172/JCI27751
Gerull B, Brodehl A (2020) Genetic animal models for arrhythmogenic cardiomyopathy. Front Physiol 11:624. https://doi.org/10.3389/fphys.2020.00624
Gittenberger-de Groot AC, Winter EM, Poelmann RE (2010) Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia. J Cell Mol Med 14:1056–1060. https://doi.org/10.1111/j.1582-4934.2010.01077.x
Giuliodori A, Beffagna G, Marchetto G, Fornetto C, Vanzi F, Toppo S, Facchinello N, Santimaria M, Vettori A, Rizzo S, Della Barbera M, Pilichou K, Argenton F, Thiene G, Tiso N, Basso C (2018) Loss of cardiac Wnt/beta-catenin signalling in desmoplakin-deficient AC8 zebrafish models is rescuable by genetic and pharmacological intervention. Cardiovasc Res 114:1082–1097. https://doi.org/10.1093/cvr/cvy057
Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld AC, Sawant AC, Kassamali B, Atsma DE, Volders PG, de Groot NM, de Boer K, Zimmerman SL, Kamel IR, van der Heijden JF, Russell SD, Jan Cramer M, Tedford RJ, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Hauer RN, Calkins H (2015) Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet 8:437–446. https://doi.org/10.1161/CIRCGENETICS.114.001003
Hariharan V, Asimaki A, Michaelson JE, Plovie E, MacRae CA, Saffitz JE, Huang H (2014) Arrhythmogenic right ventricular cardiomyopathy mutations alter shear response without changes in cell-cell adhesion. Cardiovasc Res 104:280–289. https://doi.org/10.1093/cvr/cvu212
Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML (2010) Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 49:499–507. https://doi.org/10.1016/j.yjmcc.2010.05.005
Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML (2006) Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA 103:18284–18289. https://doi.org/10.1073/pnas.0608799103
Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12:153–163. https://doi.org/10.1038/ncb2015
Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, Suh-Chin L, Aronow BJ, Tallquist MD, Molkentin JD (2016) Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7:12260. https://doi.org/10.1038/ncomms12260
Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen HS (2013) Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature 494:105–110. https://doi.org/10.1038/nature11799
Kohela A, van Kampen SJ, Moens T, Wehrens M, Molenaar B, Boogerd CJ, Monshouwer-Kloots J, Perini I, Goumans MJ, Smits AM, van Tintelen JP, van Rooij E (2021) Epicardial differentiation drives fibro-fatty remodeling in arrhythmogenic cardiomyopathy. Sci Transl Med 13:eabf2750. https://doi.org/10.1126/scitranslmed.abf2750
Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16:51–66. https://doi.org/10.1016/j.stem.2014.11.004
Lazzarini E, Jongbloed JD, Pilichou K, Thiene G, Basso C, Bikker H, Charbon B, Swertz M, van Tintelen JP, van der Zwaag PA (2015) The ARVD/C genetic variants database: 2014 update. Hum Mutat 36:403–410. https://doi.org/10.1002/humu.22765
Li D, Liu Y, Maruyama M, Zhu W, Chen H, Zhang W, Reuter S, Lin SF, Haneline LS, Field LJ, Chen PS, Shou W (2011) Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum Mol Genet 20:4582–4596. https://doi.org/10.1093/hmg/ddr392
Li J, Swope D, Raess N, Cheng L, Muller EJ, Radice GL (2011) Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol 31:1134–1144. https://doi.org/10.1128/MCB.01025-10
Lin RJ, Su ZZ, Liang SM, Chen YY, Shu XR, Nie RQ, Wang JF, Xie SL (2016) Role of circulating fibrocytes in cardiac fibrosis. Chin Med J (Engl) 129:326–331. https://doi.org/10.4103/0366-6999.174503
Liu G, Ma C, Yang H, Zhang PY (2017) Transforming growth factor beta and its role in heart disease. Exp Ther Med 13:2123–2128. https://doi.org/10.3892/etm.2017.4246
Liu J, Wang H, Zuo Y, Farmer SR (2006) Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol 26:5827–5837. https://doi.org/10.1128/MCB.00441-06
Liu Q, Huang X, Oh JH, Lin RZ, Duan S, Yu Y, Yang R, Qiu J, Melero-Martin JM, Pu WT, Zhou B (2014) Epicardium-to-fat transition in injured heart. Cell Res 24:1367–1369. https://doi.org/10.1038/cr.2014.125
Lombardi R, Chen SN, Ruggiero A, Gurha P, Czernuszewicz GZ, Willerson JT, Marian AJ (2016) Cardiac fibro-adipocyte progenitors express desmosome proteins and preferentially differentiate to adipocytes upon deletion of the desmoplakin gene. Circ Res 119:41–54. https://doi.org/10.1161/CIRCRESAHA.115.308136
Lombardi R, da Graca C-H, Bell A, Fromm RR, Willerson JT, Marian AJ (2011) Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res 109:1342–1353. https://doi.org/10.1161/CIRCRESAHA.111.255075
Lombardi R, Dong J, Rodriguez G, Bell A, Leung TK, Schwartz RJ, Willerson JT, Brugada R, Marian AJ (2009) Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res 104:1076–1084. https://doi.org/10.1161/circresaha.109.196899
Lyon RC, Mezzano V, Wright AT, Pfeiffer E, Chuang J, Banares K, Castaneda A, Ouyang K, Cui L, Contu R, Gu Y, Evans SM, Omens JH, Peterson KL, McCulloch AD, Sheikh F (2014) Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum Mol Genet 23:1134–1150. https://doi.org/10.1093/hmg/ddt508
Ma D, Wei H, Lu J, Ho S, Zhang G, Sun X, Oh Y, Tan SH, Ng ML, Shim W, Wong P, Liew R (2013) Generation of patient-specific induced pluripotent stem cell-derived cardiomyocytes as a cellular model of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 34:1122–1133. https://doi.org/10.1093/eurheartj/ehs226
Maione AS, Stadiotti I, Pilato CA, Perrucci GL, Saverio V, Catto V, Vettor G, Casella M, Guarino A, Polvani G, Pompilio G, Sommariva E (2021) Excess TGF-beta1 drives cardiac mesenchymal stromal cells to a pro-fibrotic commitment in arrhythmogenic cardiomyopathy. Int J Mol Sci. https://doi.org/10.3390/ijms22052673
Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y (1982) Right ventricular dysplasia: a report of 24 adult cases. Circulation 65:384–398. https://doi.org/10.1161/01.cir.65.2.384
Maslov M, Foianini S, Lovich M (2017) Delivery of drugs, growth factors, genes and stem cells via intrapericardial, epicardial and intramyocardial routes for sustained local targeted therapy of myocardial disease. Expert Opin Drug Deliv 14:1227–1239. https://doi.org/10.1080/17425247.2017.1292249
Matthes SA, Taffet S, Delmar M (2011) Plakophilin-2 and the migration, differentiation and transformation of cells derived from the epicardium of neonatal rat hearts. Cell Commun Adhes 18:73–84. https://doi.org/10.3109/15419061.2011.621561
Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A (2006) Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res 71:661–671. https://doi.org/10.1016/j.cardiores.2006.06.013
Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM (2014) Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 124:2921–2934. https://doi.org/10.1172/JCI74783
Pilato CA, Stadiotti I, Maione AS, Saverio V, Catto V, Tundo F, Dello Russo A, Tondo C, Pompilio G, Casella M, Sommariva E (2018) Isolation and characterization of cardiac mesenchymal stromal cells from endomyocardial bioptic samples of arrhythmogenic cardiomyopathy patients. J Vis Exp. https://doi.org/10.3791/57263
Piven OO, Winata CL (2017) The canonical way to make a heart: beta-catenin and plakoglobin in heart development and remodeling. Exp Biol Med (Maywood) 242:1735–1745. https://doi.org/10.1177/1535370217732737
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA (2000) Inhibition of adipogenesis by Wnt signaling. Science 289:950–953. https://doi.org/10.1126/science.289.5481.950
Rossini A, Frati C, Lagrasta C, Graiani G, Scopece A, Cavalli S, Musso E, Baccarin M, Di Segni M, Fagnoni F, Germani A, Quaini E, Mayr M, Xu Q, Barbuti A, DiFrancesco D, Pompilio G, Quaini F, Gaetano C, Capogrossi MC (2011) Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res 89:650–660. https://doi.org/10.1093/cvr/cvq290
Ruiz-Villalba A, Simon AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, Sanchez-Dominguez R, Segovia JC, Prosper F, Perez-Pomares JM (2015) Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol 65:2057–2066. https://doi.org/10.1016/j.jacc.2015.03.520
Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW (2011) A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res 108:51–59. https://doi.org/10.1161/CIRCRESAHA.110.233262
Sepehrkhouy S, Gho J, van Es R, Harakalova M, de Jonge N, Dooijes D, van der Smagt JJ, Buijsrogge MP, Hauer RNW, Goldschmeding R, de Weger RA, Asselbergs FW, Vink A (2017) Distinct fibrosis pattern in desmosomal and phospholamban mutation carriers in hereditary cardiomyopathies. Heart Rhythm 14:1024–1032. https://doi.org/10.1016/j.hrthm.2017.03.034
Smits AM, Dronkers E, Goumans MJ (2018) The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol Res 127:129–140. https://doi.org/10.1016/j.phrs.2017.07.020
Soliman H, Paylor B, Scott RW, Lemos DR, Chang C, Arostegui M, Low M, Lee C, Fiore D, Braghetta P, Pospichalova V, Barkauskas CE, Korinek V, Rampazzo A, MacLeod K, Underhill TM, Rossi FMV (2020) Pathogenic potential of Hic1-expressing cardiac stromal progenitors. Cell Stem Cell 26:459–461. https://doi.org/10.1016/j.stem.2020.01.023
Sommariva E, Brambilla S, Carbucicchio C, Gambini E, Meraviglia V, Dello Russo A, Farina FM, Casella M, Catto V, Pontone G, Chiesa M, Stadiotti I, Cogliati E, Paolin A, Ouali Alami N, Preziuso C, d’Amati G, Colombo GI, Rossini A, Capogrossi MC, Tondo C, Pompilio G (2016) Cardiac mesenchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy. Eur Heart J 37:1835–1846. https://doi.org/10.1093/eurheartj/ehv579
Sommariva E, Stadiotti I, Casella M, Catto V, Dello Russo A, Carbucicchio C, Arnaboldi L, De Metrio S, Milano G, Scopece A, Casaburo M, Andreini D, Mushtaq S, Conte E, Chiesa M, Birchmeier W, Cogliati E, Paolin A, Konig E, Meraviglia V, De Musso M, Volani C, Cattelan G, Rauhe W, Turnu L, Porro B, Pedrazzini M, Camera M, Corsini A, Tondo C, Rossini A, Pompilio G (2021) Oxidized LDL-dependent pathway as new pathogenic trigger in arrhythmogenic cardiomyopathy. EMBO Mol Med 13:e14365. https://doi.org/10.15252/emmm.202114365
Suffee N, Moore-Morris T, Jagla B, Mougenot N, Dilanian G, Berthet M, Proukhnitzky J, Le Prince P, Tregouet DA, Puceat M, Hatem SN (2020) Reactivation of the epicardium at the origin of myocardial fibro-fatty infiltration during the atrial cardiomyopathy. Circ Res 126:1330–1342. https://doi.org/10.1161/CIRCRESAHA.119.316251
Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL (2015) Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun 6:8701. https://doi.org/10.1038/ncomms9701
Thiene G, Corrado D, Basso C (2007) Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Orphanet J Rare Dis 2:45. https://doi.org/10.1186/1750-1172-2-45
Thiene G, Nava A, Corrado D, Rossi L, Pennelli N (1988) Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 318:129–133. https://doi.org/10.1056/NEJM198801213180301
Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S (2011) Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124:3654–3664. https://doi.org/10.1242/jcs.086629
van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC (2008) Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol 214:377–386. https://doi.org/10.1002/path.2281
van Tuyn J, Atsma DE, Winter EM, van der Velde-van DI, Pijnappels DA, Bax NA, Knaan-Shanzer S, Gittenberger-de Groot AC, Poelmann RE, van der Laarse A, van der Wall EE, Schalij MJ, de Vries AA (2007) Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 25:271–278. https://doi.org/10.1634/stemcells.2006-0366
Wang J, Liu S, Heallen T, Martin JF (2018) The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol 15:672–684. https://doi.org/10.1038/s41569-018-0063-3
Yamaguchi Y, Cavallero S, Patterson M, Shen H, Xu J, Kumar SR, Sucov HM (2015) Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARgamma activation. Proc Natl Acad Sci USA 112:2070–2075. https://doi.org/10.1073/pnas.1417232112
Yaniz-Galende E, Roux M, Nadaud S, Mougenot N, Bouvet M, Claude O, Lebreton G, Blanc C, Pinet F, Atassi F, Perret C, Dierick F, Dussaud S, Leprince P, Tregouet DA, Marazzi G, Sassoon D, Hulot JS (2017) Fibrogenic potential of PW1/Peg3 expressing cardiac stem cells. J Am Coll Cardiol 70:728–741. https://doi.org/10.1016/j.jacc.2017.06.010
Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K (2005) Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol 14:241–246. https://doi.org/10.1016/j.carpath.2005.05.004
Yuan P, Cheedipudi SM, Rouhi L, Fan S, Simon L, Zhao Z, Hong K, Gurha P, Marian AJ (2021) Single cell RNA-sequencing uncovers paracrine functions of the epicardial-derived cells in arrhythmogenic cardiomyopathy. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.052928
Zangi L, Oliveira MS, Ye LY, Ma Q, Sultana N, Hadas Y, Chepurko E, Spater D, Zhou B, Chew WL, Ebina W, Abrial M, Wang QD, Pu WT, Chien KR (2017) Insulin-like growth factor 1 receptor-dependent pathway drives epicardial adipose tissue formation after myocardial injury. Circulation 135:59–72. https://doi.org/10.1161/CIRCULATIONAHA.116.022064
Zhou B, von Gise A, Ma Q, Rivera-Feliciano J, Pu WT (2008) Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochem Biophys Res Commun 375:450–453. https://doi.org/10.1016/j.bbrc.2008.08.044
Zhurinsky J, Shtutman M, Ben-Ze’ev A (2000) Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci 113(Pt 18):3127–3139
Funding
This work was supported by the Dutch CardioVascular Alliance (DCVA), an initiative with support of the Dutch Heart Foundation, [DCVA2017-18 ARENA-PRIME].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. K. and E. v. R. filed a patent application #PCT/EP2020/051489 entitled “TFAP2 inhibition for treating cardiac disease involving fibro-fatty replacement”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kohela, A., van Rooij, E. Fibro-fatty remodelling in arrhythmogenic cardiomyopathy. Basic Res Cardiol 117, 22 (2022). https://doi.org/10.1007/s00395-022-00929-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-022-00929-4