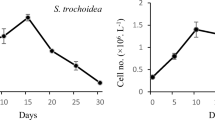
Abstract
This study elucidates the responses of bloom-forming benthic dinoflagellates, Amphidinium carterae and Bysmatrum gregarium (formerly known as B. caponii) to salinity changes and prolonged darkness. Both dinoflagellates are known to inhabit rock pools that experiences significant variations in environmental conditions. In both dinoflagellates, salinity changes did not trigger cyst formation but morphological responses were different under prolonged darkness. B. gregarium underwent encystment while A. carterae showed cell shrinkage and flagella movement (up to 9 days) without cell division. This study documents cyst formation in B. gregarium for the first time. However, both dinoflagellates showed reduced growth and photosynthetic efficiency under lower salinity (< 5) and prolonged darkness. Interestingly, both dinoflagellates did not show a maximum photosynthetic efficiency of 0.65 under optimal growth conditions which could be due to the prevalence of carotenoid-chlorophyll protein complex and diatoxanthin (fluorescence quencher). This study proposes that seasonal rainfall (e.g., Southwest monsoon along the Indian coast) can control the proliferation of both dinoflagellates in rock pools (i.e., just before the onset of monsoonal rainfall) as it hampers cell growth, inhibits photosynthesis, and does not induce cyst formation as adaptive survival strategies. Further findings on their dark survival for several days will have implications in the studies related to the transportation to different locations through the ship’s ballast water discharge or to deeper sediments in the intertidal regions.








Similar content being viewed by others
Data availability
All data concerned to the study are included in this manuscript.
References
Anglès S, Reñé A, Garcés E et al (2017) Morphological and molecular characterization of Bysmatrum subsalsum (Dinophyceae) from the western Mediterranean Sea reveals the existence of cryptic species. J Phycol 53:833–847
Aquino-Cruz A, Okolodkov YB (2016) Impact of increasing water temperature on growth, photosynthetic efficiency, nutrient consumption and potential toxicity of Amphidinium cf. carterae and Coolia monotis (Dinoflagellata). Rev Biol Mar Oceanogr 51:565–580
Barlow SB, Triemer RE (1988) Alternate life history stages in Amphidinium klebsii (Dinophyceae, Pyrrophyta). Phycologia 27:413–420
Barnett A, Méléder V, Dupuy C, Lavaud J (2020) The vertical migratory rhythm of intertidal microphytobenthos in sediment depends on the light photoperiod, intensity and spectrum: evidence for a positive effect of blue wavelengths. Front Mar Sci 7:212
Brand LE (1984) The salinity tolerance of forty-six marine phytoplankton isolates. Estuar Coast Shelf Sci 18:543–556
Cao Vien M (1967) Sur l’existence de phenomenes sexuels chez un Peridinien libre, l’Amphidinium carteri. CR Acad Sc Paris 264:1006–1008
Cao Vien M (1968) Sur la germination du zygote et sur un mode particulier de multiplication végétative chez le Péridinien libre Amphidinium carteri. C R Acad Sc Paris 267:701–703
Coleman AW (1998) Volvox: Molecular-genetic origins of multicellularity and cellular differentiation. Phycologia 37:314–315
Congleton JL (1980) Observations on the responses of some southern california tidepool fishes to nocturnal hypoxic stress. Comp Biochem Physiol -Part A Physiol 66:719–722
Dagenais-Bellefeuille S, Morse D (2013) Putting the N in dinoflagellates. Front Microbiol 4:369
Damjanović A, Ritz T, Schulten K (2000) Excitation transfer in the peridinin-chlorophyll-protein of Amphidinium carterae. Biophys J 79:1695–1705
Dubinsky Z (1992) The functional and optical absorption cross-sections of phytoplankton photosynthesis. Primary productivity and biogeochemical cycles in the sea. Springer, US, pp 31–45
Durán-Riveroll LM, Cembella AD, Okolodkov YB (2019) A review on the biodiversity and biogeography of toxigenic benthic marine dinoflagellates of the coasts of Latin America. Front Mar Sci 6:148
Falkowski PG, Raven JA (2014) Aquatic Photosynthesis. Princeton University Press
Faust MA, Steidinger KA (1998) Bysmatrum gen. nov. (Dinophyceae) and three new combinations for benthic scrippsielloid species. Phycologia 37:47–52
Franklin DJ, Berges JA (2004) Mortality in cultures of the dinoflagellate Amphidinium carterae during culture senescence and darkness. Proc R Soc B Biol Sci 271:2099–2107
Freudenthal HD (1962) Symbiodinium gen. nov. and Symbiodinium microadriaticum sp. nov., a Zooxanthella: taxonomy, life cycle and morphology. J Protozool 9:45–52
Fogg GE, Thake B (1987) Algal cultures and phytoplankton ecology. Univ of Wisconsin Press
Gottschling M, Soehner S, Zinssmeister C et al (2012) Delimitation of the Thoracosphaeraceae (Dinophyceae), including the calcareous dinoflagellates, based on large amounts of ribosomal RNA sequence data. Protist 163:15–24
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (cleve) Gran. Can J Microbiol 8:229–239
Häggqvist K, Lindholm T (2015) Phytoplankton communities in rock pools on the Åland Islands, SW Finland – environmental variables, functional groups and strategies. Biodiversity 16:15–26
Hofmann E, Wrench PM, Sharples FP et al (1996) Structural basis of light harvesting by carotenoids: peridinin- chlorophyll-protein from Amphidinium carterae. Science 80(272):1788–1791
Ismael AAH, Halim Y, Khalil AG (1999) Optimum growth conditions for Amphidinium carterae Hulburt from eutrophic waters in Alexandria (Egypt) and its toxicity to the brine shrimp Artemia salina. Grana 38:179–185
Jensen SL, Muller-Parker G (1994) Inorganic nutrient fluxes in anemone-dominated tide pools. Pac Sci 3:32–48
Jeong HJ, Jang SH, Kang NS et al (2012) Molecular characterization and morphology of the photosynthetic dinoflagellate Bysmatrum caponii from two solar saltons in western Korea. Ocean Sci J 47:1–18
Jochem FJ (1999) Dark survival strategies in marine phytoplankton assessed by cytometric measurement of metabolic activity with fluorescein diacetate. Mar Biol 135:721–728
Kaiblinger C, Dokulil MT (2006) Application of fast repetition rate fluorometry to phytoplankton photosynthetic parameters in freshwaters. Photosynth Res 88:19–30
Kim H, Bok T-H, Paeng D-G et al (2017) Mobility of Amphidinium carterae hulburt measured by high-frequency ultrasound. J Acoust Soc Am 141(1):395–401
Kim YO, Han MS (2000) Seasonal relationships between cyst germination and vegetative population of Scrippsiella trochoidea (Dinophyceae). Mar Ecol Prog Ser 204:111–118
Kirk DL (1998) Volvox: a search for the molecular and genetic origins of multicellularity and cellular differentiation (No. 33). Cambridge University Press
Kita T, Fukuyo Y, Tokuda H, Hirano R (1993) Sexual reproduction of Alexandrium hiranoi (Dinophyceae). Bull Plankt Soc Japan 39:79–85
Kolber Z, Falkowski PG (1993) Use of active fluorescence to estimate phytoplankton photosynthesis in situ. Limnol Oceanogr 38:1646–1665
Kolber Z, Zehr J, Falkowski P (1988) Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II. Plant Physiol 88:923–929
Lee JJ, Shpigel M, Freeman S et al (2003) Physiological ecology and possible control strategy of a toxic marine dinoflagellate, Amphidinium sp., from the benthos of a mariculture pond. Aquaculture 217:351–371
Ley AC, Mauzerall DC (1982) Absolute absorption cross-sections for Photosystem II and the minimum quantum requirement for photosynthesis in Chlorella vulgaris. BBA - Bioenerg 680:95–106
Limoges A, Mertens KN, Ruíz-Fernández AC, de Vernal A (2015) First report of fossilized cysts produced by the benthic Bysmatrum subsalsum (Dinophyceae) from a shallow Mexican lagoon in the Gulf of Mexico. J Phycol 51:211–215
Liu Y, Chen T, Wang X et al (2020) Variation in the photosynthetic activities of the dinoflagellate Akashiwo sanguinea during formation of resting cysts. Mar Biol 167:158
Mandal SK, Singh RP, Patel V (2011) Isolation and characterization of exopolysaccharide secreted by a toxic dinoflagellate, Amphidinium carterae Hulburt 1957 and its probable role in harmful algal blooms (HABs). Microb Ecol 62:518–527
Mauzerall D, Greenbaum NL (1989) The absolute size of a photosynthetic unit. BBA - Bioenerg 974:119–140
McLachlan J (1961) The effect of salinity on growth and chlorophyll content in representative classes of unicellular marine algae. Can J Microbiol 7:399–406
McMinn A, Martin A (2013) Dark survival in a warming world. Proc R Soc B Biol Sci 280(1755):2909
Metaxas A, Scheibling RE (1993) Community structure and organization of tidepools. Mar Ecol Prog Ser 98:187–198
Mitbavkar S, Anil AC (2004) Vertical migratory rhythms of benthic diatoms in a tropical intertidal sand flat: Influence of irradiance and tides. Mar Biol 145:9–20
Molina-Miras A, López-Rosales L, Cerón-García MC et al (2020) Acclimation of the microalga Amphidinium carterae to different nitrogen sources: potential application in the treatment of marine aquaculture effluents. J Appl Phycol 32:1075–1094
Murray SA, Kohli GS, Farrell H et al (2015) A fish kill associated with a bloom of Amphidinium carterae in a coastal lagoon in Sydney, Australia. Harmful Algae 49:19–28
Naik SM, Anil AC (2018a) Survival in the dark: strategies adopted by Tetraselmis indica (Chlorodendrophyceae, Chlorophyta). Mar Biol Res 14:448–453
Naik SM, Anil AC (2018b) Influence of darkness on pigments of Tetraselmis indica (Chlorodendrophyceae, Chlorophyta). J Photochem Photobiol B Biol 186:17–22
Patil JS, Anil AC (2019) Assessment of phytoplankton photo-physiological status from a tropical monsoonal estuary. Ecol Indic 103:289–300
Patil JS, Rodrigues RV, Paul P et al (2017) Benthic dinoflagellate blooms in tropical intertidal rock pools: elucidation of photoprotection mechanisms. Mar Biol 164:89
Polívka T, Hiller RG, Frank HA (2007) Spectroscopy of the peridinin-chlorophyll-a protein: insight into light-harvesting strategy of marine algae. Arch Biochem Biophys 458:111–120
Pommerville JC, Kochert GD (1981) Changes in somatic cell structure during senescence of Volvox carteri. Eur J Cell Biol 24:236–243
Prézelin BB (1982) Effects of light intensity on aging of the dinoflagellate Gonyaulax polyedra. Mar Biol 69:129–135
Rintala JM, Spilling K, Blomster J (2007) Temporary cyst enables long-term dark survival of Scrippsiella hangoei (Dinophyceae). Mar Biol 152:57–62
Roy R, Chitari R, Kulkarni V et al (2015) CHEMTAX-derived phytoplankton community structure associated with temperature fronts in the northeastern Arabian Sea. J Mar Syst 144:81–91
Sampayo MDM (1985) Encystment and excystment of a Portuguese isolate of Amphidinium carterae in culture. Toxic dinoflagellates. Elsevier, New York, NY, pp 125–130
Talling JF, Fogg GE (1966) Algal cultures and phytoplankton ecology. J Appl Ecol 3:215
Taylor FJR (1987) Ecology of dinoflagellates. In: The Biology of dinoflagellates. pp 399–501.
Van Heukelem L, Thomas CS, Glibert PM (2002) Sources of variability in chlorophyll analysis by fluorometry and by high performance liquid chromatography. Natl Aeronaut Sp Adm 211606:58
Watanabe MM, Fukuyo Y (1982) Encystment and excystment of red tide flagellates II. Seasonality of excystment of Protogonyaulax tamarensis and P. catenella. Res Rep Natl Inst Environ Stud 30:43–52
Zimmerman LA (2006) Environmental regulation of toxin production: comparison of hemolytic activity of Amphidinium carterae and Amphidinium klebsii. Grana 38:179–185
Acknowledgements
We are grateful to the Director of CSIR–National Institute of Oceanography for his support and encouragement. We are also grateful to Dr. AC Anil for his support, encouragement and constant guidance. The first author also acknowledges UGC, India, for Maulana Azad National Fellowships. We are also thankful to the two anonymous reviewers for their suggestions in improving the manuscript. This is an NIO contribution No. 6910.
Funding
CSIR–National Institute of Oceanography (PSC0105).
Author information
Authors and Affiliations
Contributions
RVR and JSP contributed equally to planning, data interpretation, field collection and manuscript elaboration. RVR conducted measurements and statistical analysis. JSP supervised all works and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
No animal testing was performed during this study.
Additional information
Handling Editor: Ted Harris
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodrigues, R.V., Patil, J.S. Response of benthic dinoflagellates Amphidinium carterae and Bysmatrum gregarium to salinity changes and prolonged darkness: elucidation through laboratory experiments. Aquat Ecol 56, 1113–1126 (2022). https://doi.org/10.1007/s10452-022-09960-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-022-09960-y