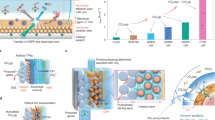
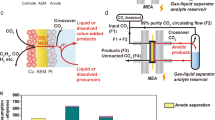
Abstract
The practical implementation of electrochemical CO2 reduction technology is greatly challenged by notable CO2 crossover to the anode side, where the crossed-over CO2 is mixed with O2, via interfacial carbonate formation in traditional CO2 electrolysers. Here we report a porous solid electrolyte reactor strategy to efficiently recover these carbon losses. By creating a permeable and ion-conducting sulfonated polymer electrolyte between cathode and anode as a buffer layer, the crossover carbonate can combine with protons generated from the anode to re-form CO2 gas for reuse without mixing with anodic O2. Using a silver nanowire catalyst for CO2 reduction to CO, we demonstrated up to 90% recovery of the crossover CO2 in an ultrahigh gas purity form (>99%), while delivering over 90% CO Faradaic efficiency under a 200 mA cm−2 current. A high continuous CO2 conversion efficiency of over 90% was achieved by recycling the recovered CO2 to the CO2 input stream.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data for the stability test shown in Fig. 6c are provided with this paper. All other data supporting this work are available from the corresponding author upon reasonable request.
References
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Aresta, M., Dibenedetto, A. & Angelini, A. Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 114, 1709–1742 (2014).
Centi, G., Quadrelli, E. A. & Perathoner, S. Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energ. Environ. Sci. 6, 1711–1731 (2013).
Olah, G. A., Prakash, G. K. S. & Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 133, 12881–12898 (2011).
Liu, X. Y. et al. Understanding trends in electrochemical carbon dioxide reduction rates. Nat. Commun. 8, 15438 (2017).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Rosen, J. et al. Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces. ACS Catal. 5, 4293–4299 (2015).
Kortlever, R., Shen, J., Schouten, K. J., Calle-Vallejo, F. & Koper, M. T. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Higgins, D., Hahn, C., Xiang, C. X., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 4, 317–324 (2019).
Ren, S. X. et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 365, 367–369 (2019).
Yan, Z. F., Hitt, J. L., Zeng, Z. C., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021).
Gu, J., Hsu, C. S., Bai, L., Chen, H. M. & Hu, X. Atomically dispersed Fe(3+) sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Wu, Y., Jiang, Z., Lu, X., Liang, Y. & Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 575, 639–642 (2019).
Delacourt, C., Ridgway, P. L., Kerr, J. B. & Newman, J. Design of an electrochemical cell making syngas (CO+H-2) from CO2 and H2O reduction at room temperature. J. Electrochem. Soc. 155, B42–B49 (2008).
Phillips, K. R., Katayama, Y., Hwang, J. & Shao-Horn, Y. Sulfide-derived copper for electrochemical conversion of CO2 to formic acid. J. Phys. Chem. Lett. 9, 4407–4412 (2018).
Sen, S., Brown, S. M., Leonard, M. & Brushett, F. R. Electroreduction of carbon dioxide to formate at high current densities using tin and tin oxide gas diffusion electrodes. J. Appl. Electrochem. 49, 917–928 (2019).
Ma, W. et al. Promoting electrocatalytic CO2 reduction to formate via sulfur-boosting water activation on indium surfaces. Nat. Commun. 10, 892 (2019).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Ma, W. C. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C-C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Choi, C. et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 3, 804–812 (2020).
Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. Nat. Commun. 9, 4614 (2018).
Pang, Y. J. et al. Efficient electrocatalytic conversion of carbon monoxide to propanol using fragmented copper. Nat. Catal. 2, 251–258 (2019).
Dinh, C. T., de Arquer, F. P. G., Sinton, D. & Sargent, E. H. High rate, selective, and stable electroreduction of CO2 to CO in basic and neutral media. ACS Energy Lett. 3, 2835–2840 (2018).
Weng, L. C., Bell, A. T. & Weber, A. Z. Towards membrane-electrode assembly systems for CO2 reduction: a modeling study. Energy Environ. Sci. 12, 1950–1968 (2019).
Kucernak, A., Bidault, F. & Smith, G. Membrane electrode assemblies based on porous silver electrodes for alkaline anion exchange membrane fuel cells. Electrochim. Acta 82, 284–290 (2012).
Hori, Y., Ito, H., Okano, K., Nagasu, K. & Sato, S. Silver-coated ion exchange membrane electrode applied to electrochemical reduction of carbon dioxide. Electrochim. Acta 48, 2651–2657 (2003).
Kim, C. et al. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc. 137, 13844–13850 (2015).
Wu, J. et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes. ACS Nano 9, 5364–5371 (2015).
Pan, Y. et al. Design of single-atom Co-N5 Catalytic Site: a robust electrocatalyst for CO2 reduction with nearly 100% CO selectivity and remarkable stability. J. Am. Chem. Soc. 140, 4218–4221 (2018).
Cheng, W. H. et al. CO2 reduction to CO with 19% efficiency in a solar-driven gas diffusion electrode flow cell under outdoor solar illumination. ACS Energy Lett. 5, 470–476 (2020).
Jeng, E. & Jiao, F. Investigation of CO2 single-pass conversion in a flow electrolyzer. React. Chem. Eng. 5, 1768–1775 (2020).
Larrazabal, G. O. et al. Analysis of mass flows and membrane cross-over in CO2 reduction at high current densities in an MEA-type electrolyzer. ACS Appl. Mater. Interfaces 11, 41281–41288 (2019).
Lin, M., Han, L. H., Singh, M. R. & Xiang, C. X. An experimental- and simulation-based evaluation of the CO2 utilization efficiency of aqueous-based electrochemical CO2 reduction reactors with ion-selective membranes. Acs Appl Energ. Mater. 2, 5843–5850 (2019).
Liu, Z. C., Yang, H. Z., Kutz, R. & Masel, R. I. CO2 electrolysis to CO and O-2 at high selectivity, stability and efficiency using sustainion membranes. J. Electrochem. Soc. 165, J3371–J3377 (2018).
Ma, M., Kim, S., Chorkendorff, I. & Seger, B. Role of ion-selective membranes in the carbon balance for CO2 electroreduction via gas diffusion electrode reactor designs. Chem. Sci. 11, 8854–8861 (2020).
Parrondo, J. et al. Degradation of anion exchange membranes used for hydrogen production by ultrapure water electrolysis. RSC Adv. 4, 9875–9879 (2014).
Patru, A., Binninger, T., Pribyl, B. & Schmidt, T. J. Design principles of bipolar electrochemical Co-electrolysis cells for efficient reduction of carbon dioxide from gas phase at low temperature. J. Electrochem. Soc. 166, F34–F43 (2019).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Reinisch, D. et al. Various CO2-to-CO electrolyzer cell and operation mode designs to avoid CO2-crossover from cathode to anode. Z. Phys. Chem. 234, 1115–1131 (2020).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C-2 products with high single-pass conversion. Joule 3, 240–256 (2019).
Zhou, X. H. et al. Solar-driven reduction of 1 atm CO2 to formate at 10% energy-conversion efficiency by use of a TiO2-Protected III-V tandem photoanode in conjunction with a bipolar membrane and a Pd/C cathode electrocatalyst. ECS Trans. 77, 31–41 (2017).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
O’Brien, C. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952 (2021).
Hatsukade, T., Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces. Phys. Chem. Chem. Phys. 16, 13814–13819 (2014).
Luan, C. H. et al. High-performance carbon dioxide electrocatalytic reduction by easily fabricated large-scale silver nanowire arrays. ACS Appl. Mater. Inter 10, 17950–17956 (2018).
Zhao, S., Jin, R. X. & Jin, R. C. Opportunities and challenges in CO2 reduction by gold- and silver-based electrocatalysts: From bulk metals to nanoparticles and atomically precise nanoclusters. ACS Energy Lett. 3, 452–462 (2018).
Bandara, T. M. et al. Effect of the alkaline cation size on the conductivity in gel polymer electrolytes and their influence on photo electrochemical solar cells. Phys. Chem. Chem. Phys. 18, 10873–10881 (2016).
Endrodi, B. et al. Operando cathode activation with alkali metal cations for high current density operation of water-fed zero-gap carbon dioxide electrolyzers. Nat. Energy 6, 439–448 (2021).
Wang, R. M. et al. Maximizing Ag utilization in high-rate CO2 electrochemical reduction with a coordination polymer-mediated gas diffusion electrode. ACS Energy Lett. 4, 2024–2031 (2019).
Fan, L., Xia, C., Zhu, P., Lu, Y. Y. & Wang, H. T. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 11, 3633 (2020).
Xia, C., Xia, Y., Zhu, P., Fan, L. & Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Yang, H., Kaczur, J. J., Sajjad, S. D. & Masel, R. I. CO2 conversion to formic acid in a three compartment cell with sustainion (TM) membranes. ECS Trans. 77, 1425–1431 (2017).
Zhu, P. et al. Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction. P Natl Acad. Sci. USA 118, e2010868118 (2021).
Matin, N. S., Remias, J. E., Neathery, J. K. & Liu, K. L. Facile method for determination of amine speciation in CO2 capture solutions. Ind. Eng. Chem. Res. 51, 6613–6618 (2012).
Pinho, C. The positive displacement method for calibration of gas flow meters. The influence of gas compressibility. Appl. Therm. Eng. 41, 111–115 (2012).
Wiebe, R. & Gaddy, V. L. The solubility of carbon dioxide in water at various temperatures from 12 to 40 degrees and at pressures to 500 atmospheres—critical phenomena. J. Am. Chem. Soc. 62, 815–817 (1940).
Jiang, K. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energ. Environ. Sci. 11, 893–903 (2018).
Zhao, C. et al. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 139, 8078–8081 (2017).
Yang, H. B. et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Zheng, T. T. et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 3, 265–278 (2019).
Jiang, K. et al. Transition-metal single atoms in a graphene shell as active centers for highly efficient artificial photosynthesis. Chem. 3, 950–960 (2017).
Gong, Q. F. et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nat. Commun. 10, 2807 (2019).
Kim, D., Kley, C. S., Li, Y. F. & Yang, P. D. Copper nanoparticle ensembles for selective electroreduction of CO2 to C-2-C-3 products. Proc. Natl Acad. Sci. USA 114, 10560–10565 (2017).
Loiudice, A. et al. Tailoring copper nanocrystals towards C-2 products in electrochemical CO2 reduction. Angew. Chem. Int Ed. 55, 5789–5792 (2016).
Acknowledgements
We acknowledge the support from Rice University, the National Science Foundation grant no. 2029442, the Welch Foundation Research grant (C-2051-2020040), and the David and Lucile Packard Foundation (grant no. 2020-71371). This work was performed in part at the Shared Equipment Authority at Rice University. We acknowledge the use of aberration-corrected scanning transmission electron microscopy coupled with electron energy loss spectroscopy at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility.
Author information
Authors and Affiliations
Contributions
H.W. supervised the project. J.Y.T.K., P.Z. and H.W. designed the research. J.Y.T.K. and P.Z. performed the research. F.-Y.C., Z.-Y.W. and D.A.C. contributed new reagents/analytic tools. J.Y.T.K., P.Z., F.-Y.C., Z.-Y.W., D.A.C. and H.W. analysed the data. J.Y.T.K., P.Z. and H.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Sarah Lamaison, Hongyan Liang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs 1−17, Note 1, Table 1 and References.
Supplementary Video 1
CO2 collection into a balloon during CO2RR in PSE reactor. 240× speed video of CO2 collection during CO2RR to CO using an Ag NW catalyst. The balloon inflation shows continuous recovery of CO2 gas during 90 min of CO2RR in a PSE reactor.
Source data
Source Data Fig. 6
Chronopotentiometry cell voltage data for long-term electrolysis test.
Rights and permissions
About this article
Cite this article
Kim, J.Y.‘., Zhu, P., Chen, FY. et al. Recovering carbon losses in CO2 electrolysis using a solid electrolyte reactor. Nat Catal 5, 288–299 (2022). https://doi.org/10.1038/s41929-022-00763-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00763-w
This article is cited by
-
Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A
Nature Energy (2024)
-
Electrochemical Carbon Dioxide Reduction in Acidic Media
Electrochemical Energy Reviews (2024)
-
Different distributions of multi-carbon products in CO2 and CO electroreduction under practical reaction conditions
Nature Catalysis (2023)
-
Selective CO2 electrolysis to CO using isolated antimony alloyed copper
Nature Communications (2023)
-
The role of ionic blockades in controlling the efficiency of energy recovery in forward bias bipolar membranes
Nature Energy (2023)