Abstract
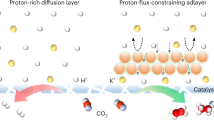
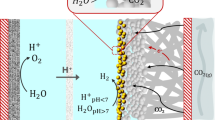
The reaction of carbon dioxide with hydroxide to form carbonate in near-neutral or alkaline medium severely limits the energy and carbon efficiency of CO2 electroreduction. Here we show that by suppression of the otherwise predominant hydrogen evolution using alkali cations, efficient CO2 electroreduction can be conducted in acidic medium, overcoming the carbonate problem. The cation effects are general for three typical catalysts including carbon-supported tin oxide, gold and copper, leading to Faradaic efficiency as high as 90% for formic acid and CO formation. Our analysis suggests that hydrated alkali cations physisorbed on the cathode modify the distribution of electric field in the double layer, which impedes hydrogen evolution by suppression of migration of hydronium ions while at the same time promoting CO2 reduction by stabilization of key intermediates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data that support the findings of this study can be found in the article and the Supplementary information. Source data are available from the corresponding author upon request. Datasets for Figs. 1–6 can be found in Zenodo53.
References
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2018).
Dinh, C.-T., García de Arquer, F. P., Sinton, D. & Sargent, E. H. High rate, selective, and stable electroreduction of CO2 to CO in basic and neutral media. ACS Energy Lett. 3, 2835–2840 (2018).
Zhang, B. A., Ozel, T., Elias, J. S., Costentin, C. & Nocera, D. G. Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on gold electrodes. ACS Cent. Sci. 5, 1097–1105 (2019).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA 113, E4585–E4593 (2016).
Varela, A. S. et al. pH effects on the selectivity of the electrocatalytic CO2 reduction on graphene-embedded Fe–N–C motifs: bridging concepts between molecular homogeneous and solid-state heterogeneous catalysis. ACS Energy Lett. 3, 812–817 (2018).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–719 (2021).
Hori, Y. in Modern Aspects of Electrochemistry (eds Vayenas, C. G. et al.) 89–189 (Springer, 2008).
Zhu, D. D., Liu, J. L. & Qiao, S. Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 28, 3423–3452 (2016).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Wang, J., Xu, F., Jin, H., Chen, Y. & Wang, Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv. Mater. 29, 1605838 (2017).
Shao, Y., Xiao, X., Zhu, Y.-P. & Ma, T.-Y. Single-crystal cobalt phosphate nanosheets for biomimetic oxygen evolution in neutral electrolytes. Angew. Chem. Int. Ed. Engl. 58, 14599–14604 (2019).
Ringe, S. et al. Double layer charging driven carbon dioxide adsorption limits the rate of electrochemical carbon dioxide reduction on Gold. Nat. Commun. 11, 33 (2020).
Bondue, C. J., Graf, M., Goyal, A. & Koper, M. T. M. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 143, 279–285 (2021).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Singh, M. R., Kwon, Y., Lum, Y., Ager, J. W. & Bell, A. T. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016).
Ringe, S. et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Liu, L.-X. et al. Tuning Sn3O4 for CO2 reduction to formate with ultra-high current density. Nano Energy 77, 105296 (2020).
Gu, J., Hsu, C.-S., Bai, L., Chen, H. M. & Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A·cm−2. Science 367, 661–666 (2020).
Ma, S. et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 301, 219–228 (2016).
Sandberg, R. B., Montoya, J. H., Chan, K. & Nørskov, J. K. CO-CO coupling on Cu facets: coverage, strain and field effects. Surf. Sci. 654, 56–62 (2016).
Zhan, C. et al. Revealing the CO coverage-driven C–C coupling mechanism for electrochemical CO2 reduction on Cu2O nanocubes via operando Raman spectroscopy. ACS Catal. 11, 7694–7701 (2021).
Kim, Y. et al. Time-resolved observation of C–C coupling intermediates on Cu electrodes for selective electrochemical CO2 reduction. Energy Environ. Sci. 13, 4301–4311 (2020).
Liu, X. et al. pH effects on the electrochemical reduction of CO2 towards C2 products on stepped copper. Nat. Commun. 10, 32 (2019).
Wuttig, A. & Surendranath, Y. Impurity ion complexation enhances carbon dioxide reduction catalysis. ACS Catal. 5, 4479–4484 (2015).
Newman, J. & Thomas-Alyea, K. E. Electrochemical Systems 3rd edn (John Wiley & Sons, 2004).
Wuttig, A., Yoon, Y., Ryu, J. & Surendranath, Y. Bicarbonate is not a general acid in Au-catalyzed CO2 electroreduction. J. Am. Chem. Soc. 139, 17109–17113 (2017).
Lee, M.-Y., Ringe, S., Kim, H., Kang, S. & Kwon, Y. Electric field mediated selectivity switching of electrochemical CO2 reduction from formate to CO on carbon supported Sn. ACS Energy Lett. 5, 2987–2994 (2020).
Vijay, S. et al. Dipole-field interactions determine the CO2 reduction activity of 2D Fe–N–C single-atom catalysts. ACS Catal. 10, 7826–7835 (2020).
Jafarzadeh, A., Bal, K. M., Bogaerts, A. & Neyts, E. C. Activation of CO2 on copper surfaces: the synergy between electric field, surface morphology, and excess electrons. J. Phys. Chem. C Nanomater Interfaces 124, 6747–6755 (2020).
Chen, L. D., Urushihara, M., Chan, K. & Nørskov, J. K. Electric field effects in electrochemical CO2 reduction. ACS Catal. 6, 7133–7139 (2016).
Ludwig, T. et al. Atomistic insight into cation effects on binding energies in Cu-catalyzed carbon dioxide reduction. J. Phys. Chem. C Nanomater Interfaces 124, 24765–24775 (2020).
Hedström, S. et al. Spin uncoupling in chemisorbed OCCO and CO2: two high-energy intermediates in catalytic CO2 reduction. J. Phys. Chem. C Nanomater Interfaces 122, 12251–12258 (2018).
Ooka, H., Figueiredo, M. C. & Koper, M. T. M. Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Langmuir 33, 9307–9313 (2017).
Thorson, M. R., Siil, K. I. & Kenis, P. J. A. Effect of cations on the electrochemical conversion of CO2 to CO. J. Electrochem. Soc. 160, F69–F74 (2012).
Zhang, Z.-Q., Banerjee, S., Thoi, V. S. & Shoji Hall, A. Reorganization of interfacial water by an amphiphilic cationic surfactant promotes CO2 reduction. J. Phys. Chem. Lett. 11, 5457–5463 (2020).
Li, J., Li, X., Gunathunge, C. M. & Waegele, M. M. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl Acad. Sci. USA 116, 9220–9229 (2019).
Bohra, D., Chaudhry, J. H., Burdyny, T., Pidko, E. A. & Smith, W. A. Modeling the electrical double layer to understand the reaction environment in a CO2 electrocatalytic system. Energy Environ. Sci. 12, 3380–3389 (2019).
Choi, C. et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 3, 804–812 (2020).
Li, J. et al. Hydroxide is not a promoter of C2+ product formation in the electrochemical reduction of CO on copper. Angew. Chem. Int. Ed. Engl. 59, 4464–4469 (2020).
Fawcett, W. R. Fifty years of studies of double layer effects in electrode kinetics—a personal view. J. Solid State Electrochem. 15, 1347 (2011).
Frumkin, A. N., Petry, O. A. & Nikolaeva-Fedorovich, N. V. On the determination of the value of the charge of the reacting particle and of the constant α from the dependence of the rate of electro-reduction on the potential and concentration of the solution. Electrochim. Acta 8, 177–192 (1963).
Fawcett, W. R. & Mackey, M. D. The electroreduction of peroxydisulphate anion in formamide. J. Electroanal. Chem. Interfac. Electrochem. 27, 219–231 (1970).
Gu, J., Héroguel, F., Luterbacher, J. & Hu, X. Densely packed, ultra small SnO nanoparticles for enhanced activity and selectivity in electrochemical CO2 reduction. Angew. Chem. Int. Ed. Engl. 57, 2943–2947 (2018).
Wang, Y. et al. Ensemble effect in bimetallic electrocatalysts for CO2 reduction. J. Am. Chem. Soc. 141, 16635–16642 (2019).
Guo, H. et al. Shape-selective formation of monodisperse copper nanospheres and nanocubes via disproportionation reaction route and their optical properties. J. Phys. Chem. C Nanomater Interfaces 118, 9801–9808 (2014).
Gu, J. et al. Data of the paper “Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium”. Zenodo https://doi.org/10.5281/zenodo.5751314 (2021).
Acknowledgements
We thank L. Bai for help with TEM characterizations, and H. Qin for help with XPS characterization and RDE tests. This work was supported by the European Research Council (no. 681292 to X.H), NCCR Catalysis (no. 180544 to X.H. and S.H.), a National Centre of Competence in Research funded by the Swiss National Science Foundation, the EPFL (X.H. and S.H.), Foundation of Shenzhen Science, Technology and Innovation Commission (no. JCYJ20210324104414039 to J.G.), the European Union Marie Sklodowska-Curie Individual Fellowships (no. 891545 to W.R.), the European Union’s Horizon 2020 research and innovation programme (no. 85144, SELECT-CO2, to S.H.) and a Starting Grant of the Swiss National Science Foundation (no. 155876 to S.H.).
Author information
Authors and Affiliations
Contributions
J.G. performed the majority of synthesis, characterization and electrochemical tests. S.L. performed simulations. W.N. performed Au RDE tests. W.R. performed electrochemical tests in near-neutral and alkaline media. J.G., S.L., S.H. and X.H. analysed data. J.G. and X.H. wrote the paper, with input from all other co-authors. S.H. and X.H. directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Notes 1 and 2, Figs. 1–26, Tables 1–4 and references.
Supplementary Video 1
RDE experiment. Au RDE in 0.1 M HOTf + 0.4 M KOTf. Current density was –200 mA cm–2 and rotating speed was 1,600 r.p.m.
Rights and permissions
About this article
Cite this article
Gu, J., Liu, S., Ni, W. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat Catal 5, 268–276 (2022). https://doi.org/10.1038/s41929-022-00761-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00761-y
This article is cited by
-
Single-atom tailored atomically-precise nanoclusters for enhanced electrochemical reduction of CO2-to-CO activity
Nature Communications (2024)
-
Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A
Nature Energy (2024)
-
Nanocurvature-induced field effects enable control over the activity of single-atom electrocatalysts
Nature Communications (2024)
-
Acidic media enables oxygen-tolerant electrosynthesis of multicarbon products from simulated flue gas
Nature Communications (2024)
-
Acid–base chemistry and the economic implication of electrocatalytic carboxylate production in alkaline electrolytes
Nature Catalysis (2024)