Abstract
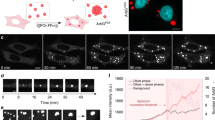
Biomolecular condensates organize biochemistry, yet little is known about how cells control the position and scale of these structures. In cells, condensates often appear as relatively small assemblies that do not coarsen into a single droplet despite their propensity to fuse. Here, we report that ribonucleoprotein condensates of the glutamine-rich protein Whi3 interact with the endoplasmic reticulum, which prompted us to examine how membrane association controls condensate size. Reconstitution revealed that membrane recruitment promotes Whi3 condensation under physiological conditions. These assemblies rapidly arrest, resembling size distributions seen in cells. The temporal ordering of molecular interactions and the slow diffusion of membrane-bound complexes can limit condensate size. Our experiments reveal a trade-off between locally enhanced protein concentration at membranes, which favours condensation, and an accompanying reduction in diffusion, which restricts coarsening. Given that many condensates bind endomembranes, we predict that the biophysical properties of lipid bilayers are key for controlling condensate sizes throughout the cell.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Data analysis was conducted using particle analysis tools in ImageJ (https://imagej.net/imaging/particle-analysis), cmeAnalysis software (https://github.com/DanuserLab/cmeAnalysis), particle tracking software (http://site.physics.georgetown.edu/matlab/) and msdanalyzer software (https://tinevez.github.io/msdanalyzer/). No custom code was generated for this study.
References
Banani, S., Lee, H., Hyman, A. & Rosen, M. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Berry, J., Brangwynne, C. P. & Haataja, M. Physical principles of intracellular organization via active and passive phase transitions. Rep. Prog. Phys. 81, 046601 (2018).
Brangwynne, C., Tompa, P. & Pappu, R. Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Berry, J., Weber, S. C., Vaidya, N., Haataja, M. & Brangwynne, C. P. RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl Acad. Sci. USA 112, E5237–E5245 (2015).
Stanich, C. A. et al. Coarsening dynamics of domains in lipid membranes. Biophys. J. 105, 444–454 (2013).
Weber, C. A., Zwicker, D., Julicher, F. & Lee, C. F. Physics of active emulsions. Rep. Prog. Phys. 82, 064601 (2019).
Lee, D. S. W., Wingreen, N. S. & Brangwynne, C. P. Chromatin mechanics dictates subdiffusion and coarsening dynamics of embedded condensates. Nat. Phys. 17, 531–538 (2021).
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012).
Elbaum-Garfinkle, S. et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl Acad. Sci. USA 112, 7189–7194 (2015).
Lin, Y., Protter, D. S., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Zhang, H. et al. RNA controls polyQ protein phase transitions. Mol. Cell 60, 220–230 (2015).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Banani, S. F. et al. Compositional control of phase-separated cellular bodies. Cell 166, 651–663 (2016).
Yamazaki, T. et al. Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. Mol. Cell 70, 1038–1053.e7 (2018).
Lee, J. E., Cathey, P. I., Wu, H., Parker, R. & Voeltz, G. K. Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367, eaay7108 (2020).
Case, L. B., Ditlev, J. A. & Rosen, M. K. Regulation of transmembrane signaling by phase separation. Annu. Rev. Biophys. 48, 465–494 (2019).
Zhao, Y. G. & Zhang, H. Phase separation in membrane biology: the interplay between membrane-bound organelles and membraneless condensates. Dev. Cell 55, 30–44 (2020).
Ditlev, J. A. Membrane-associated phase separation: organization and function emerge from a two-dimensional milieu. J. Mol. Cell Biol. 13, 319–324 (2021).
Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016).
Huang, W. Y. et al. Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS. Proc. Natl Acad. Sci. USA 113, 8218–8223 (2016).
Huang, W. Y. C. et al. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363, 1098–1103 (2019).
Ditlev, J. A. et al. A composition-dependent molecular clutch between T cell signaling condensates and actin. eLife 8, e42695 (2019).
Chung, J. K. et al. Coupled membrane lipid miscibility and phosphotyrosine-driven protein condensation phase transitions. Biophys. J. 120, 1257–1265 (2021).
Banjade, S. & Rosen, M. K. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife 3, e04123 (2014).
Case, L. B., Zhang, X., Ditlev, J. A. & Rosen, M. K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 363, 1093–1097 (2019).
Milovanovic, D., Wu, Y. M., Bian, X. & De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 361, 604–607 (2018).
Wu, X. et al. RIM and RIM-BP form presynaptic active-zone-like condensates via phase separation. Mol. Cell 73, 971–984.e5 (2019).
Day, K. J. et al. Liquid-like protein interactions catalyse assembly of endocytic vesicles. Nat. Cell Biol. 23, 366–376 (2021).
Baumann, S., Pohlmann, T., Jungbluth, M., Brachmann, A. & Feldbrugge, M. Kinesin-3 and dynein mediate microtubule-dependent co-transport of mRNPs and endosomes. J. Cell Sci. 125, 2740–2752 (2012).
Gopal, P. P., Nirschl, J. J., Klinman, E. & Holzbaur, E. L. F. Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proc. Natl Acad. Sci. USA 114, E2466–E2475 (2017).
Liao, Y. C. et al. RNA granules hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. Cell 179, 147–164.e20 (2019).
Fujioka, Y. et al. Phase separation organizes the site of autophagosome formation. Nature 578, 301–305 (2020).
Agudo-Canalejo, J. et al. Wetting regulates autophagy of phase-separated compartments and the cytosol. Nature 591, 142–146 (2021).
Ma, W. R. & Mayr, C. A membraneless organelle associated with the endoplasmic reticulum enables 3′ UTR-mediated protein–protein interactions. Cell 175, 1492–1506.e19 (2018).
Alenquer, M. et al. Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nat. Commun. 10, 19 (2019).
Snead, W. T. & Gladfelter, A. S. The control centers of biomolecular phase separation: how membrane surfaces, PTMs, and active processes regulate condensation. Mol. Cell 76, 295–305 (2019).
Lee, C. et al. Protein aggregation behavior regulates cyclin transcript localization and cell-cycle control. Dev. Cell 25, 572–584 (2013).
Lee, C., Occhipinti, P. & Gladfelter, A. S. PolyQ-dependent RNA–protein assemblies control symmetry breaking. J. Cell Biol. 208, 533–544 (2015).
Langdon, E. M. et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 360, 922–927 (2018).
Gerbich, T. M. et al. Phosphoregulation provides specificity to biomolecular condensates in the cell cycle and cell polarity. J. Cell Biol. 219, e201910021 (2020).
Aguet, F., Antonescu, C., Mettlen, M., Schmid, S. & Danuser, G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell 26, 279–291 (2013).
Przybylo, M. et al. Lipid diffusion in giant unilamellar vesicles is more than 2 times faster than in supported phospholipid bilayers under identical conditions. Langmuir 22, 9096–9099 (2006).
Beckers, D., Urbancic, D. & Sezgin, E. Impact of nanoscale hindrances on the relationship between lipid packing and diffusion in model membranes. J. Phys. Chem. B 124, 1487–1494 (2020).
Tanaka, H. Viscoelastic phase separation. J. Phys. Condens. Matter 12, R207–R264 (2000).
Mitchison, T. J. Beyond Langmuir: surface-bound macromolecule condensates. Mol. Biol. Cell 31, 2502–2508 (2020).
Alshareedah, I., Thurston, G. M. & Banerjee, P. R. Quantifying viscosity and surface tension of multicomponent protein–nucleic acid condensates. Biophys. J. 120, 1161–1169 (2021).
Reid, D. W. & Nicchitta, C. V. Diversity and selectivity in mRNA translation on the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 16, 221–231 (2015).
Houser, J. et al. The impact of physiological crowding on the diffusivity of membrane bound proteins. Soft Matter 12, 2127–2134 (2016).
Zwicker, D., Hyman, A. A. & Julicher, F. Suppression of Ostwald ripening in active emulsions. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 92, 012317 (2015).
Wurtz, J. D. & Lee, C. F. Chemical-reaction-controlled phase separated drops: formation, size selection, and coarsening. Phys. Rev. Lett. 120, 078102 (2018).
Bressloff, P. C. Active suppression of Ostwald ripening: beyond mean-field theory. Phys. Rev. E 101, 042804 (2020).
Ranganathan, S. & Shakhnovich, E. I. Dynamic metastable long-living droplets formed by sticker-spacer proteins. eLife 9, e56159 (2020).
Seim, I. et al. Negative regulation of a ribonucleoprotein condensate driven by dilute phase oligomerization. Preprint at bioRxiv https://doi.org/10.1101/2021.04.19.440511 (2021).
Ruff, K. M., Dar, F. & Pappu, R. V. Ligand effects on phase separation of multivalent macromolecules. Proc. Natl Acad. Sci. USA 118, e2017184118 (2021).
Zeng, M. et al. Reconstituted postsynaptic density as a molecular platform for understanding synapse formation and plasticity. Cell 174, 1172–1187 e1116 (2018).
Siahaan, V. et al. Kinetically distinct phases of tau on microtubules regulate kinesin motors and severing enzymes. Nat. Cell Biol. 21, 1086–1092 (2019).
Tan, R. et al. Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 21, 1078–1085 (2019).
Larson, A. G. et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547, 236–240 (2017).
Strom, A. R. et al. Phase separation drives heterochromatin domain formation. Nature 547, 241–245 (2017).
Gibson, B. A. et al. Organization of chromatin by intrinsic and regulated phase separation. Cell 179, 470–484.e21 (2019).
Keenen, M. M. et al. HP1 proteins compact DNA into mechanically and positionally stable phase separated domains. eLife 10, e64563 (2021).
Ruiz-Taylor, L. et al. Monolayers of derivatized poly(l-lysine)-grafted poly(ethylene glycol) on metal oxides as a class of biomolecular interfaces. Proc. Natl Acad. Sci. USA 98, 852–857 (2001).
Snead, W. T. et al. BAR scaffolds drive membrane fission by crowding disordered domains. J. Cell Biol. 218, 664–682 (2019).
Tarantino, N. et al. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO-IKK supramolecular structures. J. Cell Biol. 204, 231–245 (2014).
Lee, H. B. et al. Rotational and translational diffusion of size-dependent fluorescent probes in homogeneous and heterogeneous environments. Phys. Chem. Chem. Phys. 20, 24045–24057 (2018).
Acknowledgements
A.S.G. acknowledges funding from the National Institutes of Health (R01-GM081506), the Howard Hughes Medical Institute (HHMI) Faculty Scholars program and the Air Force Office of Scientific Research (FA9550-20-1-0241). W.T.S. acknowledges the support of a Ruth L. Kirschstein NRSA Postdoctoral Fellowship from the National Institutes of Health (F32-GM136055). We thank C. Roden of the Gladfelter Lab for cloning the CLN3 plasmid and performing in vitro RNA transcription; C. Hayden (UT Austin, Austin, TX, USA) for discussions on image analysis; S. Di Talia (Duke University, Durham, NC, USA) for critical reading of the manuscript; T. Perdue and staff at the UNC Biology Department Imaging Core for assistance with FCS and use of the Zeiss LSM 880 microscope; and G. Danuser and S. Schmid (UT Southwestern, Dallas, TX, USA) for freely providing cmeAnalysis particle detection software.
Author information
Authors and Affiliations
Contributions
W.T.S., A.P.J., T.M.G. and A.S.G. designed the experiments. W.T.S., A.P.J., T.M.G., I.S. and Z.H. performed experiments and analysed data. W.T.S., A.P.J., I.S. and A.S.G. wrote the manuscript. All authors consulted on manuscript preparation and editing.
Corresponding author
Ethics declarations
Competing interests
A.S.G. is a scientific advisor for Dewpoint Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Jonathon Ditlev, Aurélien Roux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Quantifying Whi3-ER co-localization.
(a-d) Images of an Ashbya hypha expressing Whi3-tdTomato (tagged endogenously) and ER marker Sec63-GFP (plasmid expression). Images show merged channels (a), detected Whi3 puncta after particle detection (b), the same detected puncta overlaid with the ER channel (c), and a random distribution of the same number of puncta overlaid with the ER channel (d). (e) Local intensity in the ER channel at the Whi3 puncta shown in the above images, expressed as a fraction of the median intensity of the ER channel throughout the hypha after masking and background subtraction. Blue points: observed Whi3 positions, orange points: randomized Whi3 positions corresponding to the puncta in image (d). n = 16 puncta. Dashed line indicates the threshold for co-localization, corresponding to the median intensity of the ER channel. Black horizontal bars represent mean, vertical bars represent first s.d. p-value from two-tailed, unpaired Student’s t-test. (f) Ratio of the average local intensity in the ER channel at detected Whi3 puncta within the above hypha relative to randomized Whi3 puncta positions. A value greater than one indicates that the local intensity within the ER channel is greater on average at detected Whi3 puncta compared to randomized puncta. Each data point represents the ratio to one of n = 50 random distributions. The mean, indicated by the black horizontal bar, corresponds to one of the 60 data points in Fig. 1c. Vertical bar represents first s.d.
Extended Data Fig. 2 Tracking Whi3 puncta and ER co-localization.
(a) Time-lapse montages of Whi3 puncta associated with ER, including ER tubules (yellow arrowheads) and nuclear-associated ER (blue arrowheads). White dashed lines in first frame indicate cell periphery. Similar to Fig. 1d. (b) First frame from time-lapse shown in Supplementary Video 1. Yellow arrows indicate puncta that appear co-localized with the ER but moved out of the imaging plane during the movie and were not included in the tracking. (c) ER channel from the image in (b) with overlaid Whi3 tracks, colored according to the fraction of the track lifetime spent co-localized with the ER. All tracks clearly co-localize with ER structures. Not all tracks begin in the indicated frame. (d) Relative, local intensity in the ER channel as a function of time for the Whi3 tracks shown in (c), expressed as a fraction of the median intensity in the ER channel throughout the cell. Values greater than one (red region) were defined as co-localized with the ER. In this example, all tracks spend 100% of the lifetime co-localized with the ER. (e) Histogram of the tracks in (c-d), binned according to the fraction of track lifetime co-localized with the ER (similar to Fig. 1f). n = 7 tracked Whi3 puncta in this representative example from 60 hyphae. (f) Average intensity of all tracked Whi3 puncta as a function of the fraction of the track lifetime spent associated with the ER. Data points show moving average of the raw data, with lifetime fraction increments of 0.2. Data are mean ± 95% c.i. n = 83, 37, 45, 69, and 535 tracks in bins centered at 0.1, 0.3, 0.5, 0.7 and 0.9, respectively.
Extended Data Fig. 3 Particle detection and single molecule calibration.
(a) TIRF image of 50 pM Whi3-Atto488 on a plasma-cleaned glass coverslip. Right image shows detected puncta from cmeAnalysis software. (b) Histogram of detected puncta intensities, obtained from fits to a two-dimensional Gaussian function with standard deviation equal to the microscope PSF. The indicated peak value (red arrow) was taken as the average intensity of a single Whi3 protein. n = 3,682 puncta from a representative example of 10 independent experiments. (c-d) This single molecule intensity estimate was validated using photobleaching measurements. (c) TIRF time-lapse of 50 pM Whi3-Atto488 puncta on glass at the indicated times. Images were acquired with the same TIRF angle, laser power, and camera exposure settings used for acquisition of single molecule calibration images in (a-b). (d) Average, background-subtracted peak intensities of the puncta in the indicated colored circles in (c). Each puncta bleaches to the level of the camera background in a single step, indicating that each puncta corresponds to a single Whi3-Atto488 protein. The average, pre-bleach intensity of each puncta was comparable to our estimate of the single molecule intensity from particle detection in (b).
Extended Data Fig. 4 Membrane binding drives Whi3 condensate assembly.
(a) Time-lapse images of SLBs composed of DOPC alone (top row) or 90 mol% DOPC + 10 mol% DOPS (lower row) at the indicated times after addition of 50 nM Whi3. Images show that Whi3 puncta non-specifically interact with SLBs, but macroscopic condensates do not form. (b) FRAP reveals rapid and complete recovery of the fluorescent lipid Texas Red (TR)-DHPE in SLBs. Plot shows normalized lipid intensity within bleached region as a function of time after bleaching. Black line shows fit to single-component exponential recovery model, with recovery time constant (τ) indicated. Data are mean ± first s.d., n = 4 bleached regions from 1 SLB. (c) Confocal section (top) and maximum intensity projection (bottom) of membrane-associated condensates assembled on a GUV after addition of 400 nM Whi3. (d) Time-lapse of membrane-associated Whi3 condensates on an SLB (top row, TIRF images) and on the top of a GUV (lower row, confocal section). Red arrows indicate contacting condensates which do not fuse or round. (e) Images of rapid and complete unbinding of membrane-bound 6his-GFP at the indicated times after addition of 10 mM EDTA. Images at 1 and 5 min are contrasted equally. Plot shows GFP intensity as a function of time after EDTA addition. Data are mean ± first s.d., n = 160,000 pixel intensity values from time-lapse images of 1 SLB. (f) Solution droplets formed with 41 µM Whi3 at the indicated times after assembly, induced by lowering the KCl concentration to 75 mM. Images are maximum intensity projections from confocal z-stacks. All images contrasted equally. (g) Average radius of solution droplets as a function of time after assembly, formed with 41 µM Whi3. Black line shows fitted power law function with indicated scaling exponent. Data are mean ± first s.d. n = 439–1,171 droplets per data point, from 3 biologically independent samples. Exact n per data point provided in Source Data Extended Data Fig. 4g. (h) Time-lapse of rapid droplet fusion, 4 h after assembly. GUV and SLB membrane composition: 96 mol% DOPC, 4 mol% DGS NTA-Ni; 0.03 mol% TR-DHPE included for FRAP.
Extended Data Fig. 5 RNA is clustered at the edges of pre-formed condensates.
(a) Time series of two example condensates formed in the presence of 50 nM Whi3 and 100 pM CLN3. Images show condensates formed in proximity to an RNA puncta (black dashed box) and with no associated RNA (gray dashed box). (b) Time-lapse of condensate assembly on SLB with 50 nM Whi3 and 100 pM CLN3. Frames span 2.5–8.5 min after addition of Whi3, 1 min between frames. White arrows indicate Whi3 condensates visible after 3.5 min, and yellow arrowheads indicate condensate edge-associated CLN3 puncta 1 min later. White asterisks in final frames indicate bright CLN3 clusters that continued to assemble at condensate edges. (c) Intensity histograms of CLN3 puncta adsorbed to condensate edges or in the dilute phase on the surrounding membrane, 20–30 min after addition of 50 nM Whi3. n = 467 and 24,954 edge-adsorbed and dilute phase puncta, respectively, from 4 biologically independent samples. p-value from two-sided Kolmogorov-Smirnov test. (d) Membrane-associated condensates do not attain measurable height. Maximum intensity projections from a spinning disc confocal z-stack of condensates formed in the presence of 500 nM Whi3, with z-spacing of 0.2 µm. SLB membrane composition: 96 mol% DOPC, 4 mol% DGS NTA-Ni.
Extended Data Fig. 6 Membrane-tethered RNA recruits Whi3 and forms condensates.
(a) Confocal slices of ER and CLN3 smFISH in Ashbya cells. Arrows indicate example CLN3 puncta showing ER co-localization. (b) Images of CLN3 puncta tethered to membrane (left) or PEG surface (right) prior to addition of Whi3. Images contrasted equally. (c) Initial densities of CLN3 puncta on membranes or PEG surfaces prior to addition of Whi3. Black bars show mean ± first s.d., n = 90 and 60 images from 18 and 12 independent membrane and PEG experiments, respectively. (d) Average CLN3 puncta intensity on membrane or PEG surfaces as a function of time after addition of 50 nM Whi3. PEG-tethered CLN3 did not recruit Whi3 or cluster to the same extent as membrane-tethered CLN3, despite the higher initial CLN3 density on PEG surfaces. Data are mean ± 95% c.i. n = 1,059–1,335 and 1,804–1,987 puncta per data point in membrane and PEG experiments, respectively, from 3 biologically independent samples in each experiment. Exact n per data point provided in Source Data Extended Data Fig. 6d. (e) Histograms of scaled Whi3 puncta intensity from Ashbya cells and membrane-tethered CLN3 experiments after clustering by 50 nM Whi3. Scaled distributions obtained by dividing by the distribution means. n = 13,198 and 867 Whi3 puncta in membrane-tethered CLN3 experiment and in live cells, respectively. (f) Membrane-tethered RNA condensates 15 min after exposure to Whi3 at the indicated concentrations. CLN3 and Whi3 channels contrasted equally in all images. (g-h) Intensity of CLN3 (g) and Whi3 (h) within membrane-tethered RNA condensates as a function of bulk Whi3 concentration. Data are mean ± 95% c.i. in (g-h). Each data point in (g-h) contains n = 7,484–35,945 and 9,238–37,992 puncta in membrane and PEG experiments, respectively, from 3 biologically independent samples in each experiment. Exact n per data point provided in Source Data Extended Data Fig. 6g,h. SLB membrane composition: 99 mol% DOPC, 1 mol% DOPE cap-biotin.
Extended Data Fig. 7 Average FCS traces of solution and membrane-bound GFP.
Black curves indicate fits to single-component diffusion model. n = 10 and 27 FCS traces in solution and membrane experiments, respectively. SLB membrane composition: 96 mol% DOPC, 4 mol% DGS NTA-Ni.
Supplementary information
Supplementary Video 1
Whi3 assemblies are tethered to the ER in live Ashbya cells. Movie shows spinning disk confocal time-lapse of an Ashbya hypha expressing Whi3–tdTomato (magenta) and the ER marker Sec63–GFP (green). ER channel on the left, Whi3 channel in the centre, and merged channels on the right, 30 s per frame.
Supplementary Video 2
Recruitment of Whi3 directly to a membrane surface drives rapid assembly of protein-only condensates. Movie shows TIRF microscopy time-lapse of planar Whi3 condensates forming on SLB following the addition of 50 nM Whi3, no RNA, 30 s per frame. SLB membrane composition: 4% DGS NTA-Ni, 96% DOPC.
Supplementary Video 3
RNA is recruited from solution by membrane-bound Whi3 and forms bright clusters on the surrounding membrane outside condensates. First, 100 pM of Cy3-labelled CLN3 RNA (magenta) was added to the well, followed by 50 nM of Whi3–Atto488 (green). Movie shows TIRF microscopy time-lapse of planar Whi3 condensates forming on SLB. Whi3 channel on the left, CLN3 channel in the centre, and merged channels on the right, 30 s per frame. SLB membrane composition: 4% DGS NTA-Ni, 96% DOPC.
Supplementary Video 4
RNA recruited from solution by membrane-bound Whi3 is clustered at condensate edges. First, 100 pM of Cy3-labelled CLN3 RNA (magenta) was added to the experiment well, followed by 50 nM of Whi3–Atto488 (green). Movie shows TIRF microscopy time-lapse of planar Whi3 condensates forming on SLB. Whi3 channel on the left, CLN3 channel in the centre, and merged channels on the right, 30 s per frame. SLB membrane composition: 4% DGS NTA-Ni, 96% DOPC.
Supplementary Video 5
Membrane-tethered CLN3 RNA recruits Whi3 from solution and rapidly clusters into punctate condensates. CLN3 RNA (magenta) was first tethered to the membrane at a density of approximately 4.6 puncta 10 µm–2, and 50 nM Whi3 protein (green) was added in solution. Movie shows TIRF microscopy time-lapse of punctate Whi3–CLN3 condensates forming on SLB after addition of Whi3. CLN3 channel on the left, Whi3 channel in the centre, and merged channels on the right, 30 s per frame. SLB membrane composition: 1% DOPE cap-biotin, 99% DOPC.
Supplementary Video 6
Immobile, PEG surface-tethered CLN3 RNA does not recruit Whi3 to the same extent as membrane-tethered CLN3 and does not cluster. CLN3 RNA (magenta) was first tethered to the PEG surface at a density of approximately 7.4 puncta 10 µm–2, and 50 nM Whi3 protein (green) was added in solution. Movie shows TIRF microscopy time-lapse of CLN3 puncta on PEG surface after addition of Whi3. CLN3 channel on the left, Whi3 channel in the centre, and merged channels on the right, 30 s per frame.
Source data
Source Data Fig. 1
Numerical source data.
Source Data Fig. 2
Numerical source data.
Source Data Fig. 3
Numerical source data.
Source Data Fig. 4
Numerical source data.
Source Data Fig. 5
Numerical source data.
Source Data Fig. 6
Numerical source data.
Source Data Extended Data Fig. 1
Numerical source data.
Source Data Extended Data Fig. 2
Numerical source data.
Source Data Extended Data Fig. 3
Numerical source data.
Source Data Extended Data Fig. 4
Numerical source data.
Source Data Extended Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 6
Numerical source data.
Source Data Extended Data Fig. 7
Numerical source data.
Rights and permissions
About this article
Cite this article
Snead, W.T., Jalihal, A.P., Gerbich, T.M. et al. Membrane surfaces regulate assembly of ribonucleoprotein condensates. Nat Cell Biol 24, 461–470 (2022). https://doi.org/10.1038/s41556-022-00882-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-022-00882-3
This article is cited by
-
Phase separation-mediated biomolecular condensates and their relationship to tumor
Cell Communication and Signaling (2024)
-
Mesoscale condensates organize the cytoplasm
Nature Cell Biology (2024)
-
Biomolecular condensates create phospholipid-enriched microenvironments
Nature Chemical Biology (2024)
-
Biomolecular condensation orchestrates clathrin-mediated endocytosis in plants
Nature Cell Biology (2024)
-
VAPB-mediated ER-targeting stabilizes IRS-1 signalosomes to regulate insulin/IGF signaling
Cell Discovery (2023)