Abstract
Introduction
Biological therapies are valuable treatments for severe psoriasis. Children aged under 12 years are underrepresented in therapeutic trials for these drugs. The objective of the ‘BiPe Jr’ cohort study was to evaluate the drug survival, effectiveness, tolerance and switching patterns of biological therapies in children under 12 years of age with psoriasis.
Methods
We conducted a multicentre retrospective study of children with psoriasis who received at least one injection of a biological agent, even off-licence, before the age of 12 years in France and Italy, collecting the data between April and August 2021. The data collected were from March 2012 up to August 2021.
Results
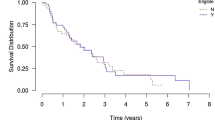
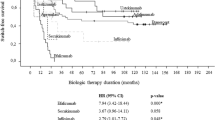
In total, 82 children (mean age: 9.1 years; females: 61.0%) received 106 treatments. The drugs administered were adalimumab (n = 49), etanercept (n = 37), ustekinumab (n = 15), anakinra (n = 2), infliximab (n = 2) and secukinumab (n = 1). The most common form of psoriasis was plaque psoriasis (62.9%). The Physician Global Assessment and the Psoriasis Area Severity Index (PASI) scores decreased significantly from baseline to 3 months after treatment initiation for the three main biological drugs; PASI went from 14.1 ± 9.4 to 4.1 ± 11.3 for adalimumab (p = 0.001), 14.9 ± 9.3 to 5.1 ± 4.0 for etanercept (p = 0.002) and 11.6 ± 8.3 to 2.6 ± 2.2 for ustekinumab (p = 0.007). A trend towards higher 2-year maintenance rates was observed for ustekinumab and adalimumab, compared with etanercept (p = 0.06). 52 children discontinued their biological therapy, most frequently due to inefficacy (n = 28) and remission (n = 14). Seven serious adverse events (SAEs) were reported, including four severe infections.
Discussion
Our analyses of drug survival and treatment patterns, combined with those of previous studies conducted in older children, indicate that there is a trend towards higher 2-year survival rates of ustekinumab and adalimumab. The SAEs identified were rare, but highlight the need for increased vigilance concerning infections. Overall, the biological therapies showed good effectiveness and safety profiles when used in daily practice for the treatment of young children with psoriasis.


Similar content being viewed by others
References
Nast A, Gisondi P, Ormerod AD, et al. European S3—guidelines on the systemic treatment of psoriasis vulgaris—update 2015–Short version—EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29:2277–94.
Mahé E, Amy-De La-Bretêque M, Phan C. Perspectives on the pharmacological management of psoriasis in pediatric and adolescent patients. Expert Rev Clin Pharmacol. 2021;14:807–19.
Menter A, Cordoro KM, Davis DMR, et al. Joint American academy of dermatology-national psoriasis foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161–201.
Eisert L, Augustin M, Bach S, et al. S2k guidelines for the treatment of psoriasis in children and adolescents—short version part 2. J Dtsch Dermatol Ges. 2019;17:959–73.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group. Eur J Pediatr. 2017;176:1339–54.
Charbit L, Mahé E, Phan A, et al. Systemic treatments in childhood psoriasis: a French multicentre study on 154 children. Br J Dermatol. 2016;174:1118–21.
Bronckers IMGJ, Seyger MMB, West DP, et al. Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147–57.
Mahé E, Corgibet F, Maccari F, et al. Prescriptions hors AMM (autorisation de mise sur le marché) dans le psoriasis de l’enfant. Ann Dermatol Venereol. 2020;147:429–38.
Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241–51.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594–603.
Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40–9.
Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35:938–47.
Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664–72.
Phan C, Beauchet A, Burztejn AC, et al. Biological treatments for paediatric psoriasis: a retrospective observational study on biological drug survival in daily practice in childhood psoriasis. J Eur Acad Dermatol Venereol. 2019;33:1984–92.
Lamer A, Laurent G, Pelayo S, et al. Exploring patient path through Sankey diagram: a proof of concept. Stud Health Technol Inform. 2020;270:218–22.
Roux J. Parcours de soins des patients atteints de sclérose en plaques à partir des données médico-administratives en France. 2018. https://tel.archives-ouvertes.fr/tel-02379451.
Wan J, Shin DB, Gelfand JM. Treatment utilization and drug survival of systemic medications among commercially insured children with psoriasis. Pediatr Dermatol. 2021;38:1169–77.
Di Lernia V, Bianchi L, Guerriero C, et al. Adalimumab in severe plaque psoriasis of childhood: a multi-center, retrospective real-life study up to 52 weeks observation. Dermatol Ther. 2019;32:e13091.
Di Lernia V, Guarneri C, Stingeni L, et al. Effectiveness of etanercept in children with plaque psoriasis in real practice: a one-year multicenter retrospective study. J Dermatolog Treat. 2017;18:1–3.
Phan C, Beauchet A, Burztejn AC, et al. Evaluation of children with psoriasis from the BiPe cohort: are patients using biotherapies in real life eligible for phase III clinical studies? Paediatr Drugs. 2019;21:169–75.
Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280–7.
Richard MA; Groupe de recherche sur le psoriasis de la Société Française de Dermatologie. Psoriasis: évaluation initiale et bilan thérapeutique pratique. Ann Dermatol Venereol. 2019;146:440–9.
Mahé E, Reguiai Z, Barthelemy H, et al. Evaluation of risk factors for body weight increment in psoriatic patients on infliximab: a multicentre, cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:151–9.
Wu MY, Yu CL, Yang SJ, Chi CC. Change in body weight and body mass index in psoriasis patients receiving biologics: a systematic review and network meta-analysis. J Am Acad Dermatol. 2020;82:101–9.
Mazhar F, Battini V, Pozzi M, et al. Changes in anthropometric parameters after anti-TNFα therapy in inflammatory bowel disease: a systematic review and meta-analysis. BioDrugs. 2020;34:649–68.
Phan C, Beauchet A, Reguiai Z, et al. Switching biologics in children with psoriasis: results from the BiPe cohort. Pediatr Dermatol. 2022;39:35–41.
Bonifati C, Morrone A, Cristaudo A, Graceffa D. Effectiveness of anti-interleukin 23 biologic drugs in psoriasis patients who failed anti-interleukin 17 regimens. A real-life experience. Dermatol Ther. 2021;34:e14584.
Sherman S, Solomon Cohen E, Amitay-Laish I, et al. IL-17A inhibitor switching—efficacy of ixekizumab following secukinumab failure. A single-center experience. Acta Derm Venereol. 2019;99:769–73.
Chiricozzi A, Conti A, Burlando M, et al. Switching from secukinumab to ustekinumab in psoriasis patients: results from a multicenter experience. Dermatol Basel Switz. 2019;235:213–8.
Damiani G, Conic RRZ, de Vita V, et al. When IL-17 inhibitors fail: Real-life evidence to switch from secukinumab to adalimumab or ustekinumab. Dermatol Ther. 2019;32:e12793.
Tai YC, Tsai TF. Switching biologics in psoriasis—practical guidance and evidence to support. Expert Rev Clin Pharmacol. 2020;13:493–503.
Badaoui A, Tounian P, Mahé E. Psoriasis and metabolic and cardiovascular comorbidities in children: a systematic review. Arch Pediatr. 2019;26:86–94.
Phan K, Lee G, Fischer G. Pediatric psoriasis and association with cardiovascular and metabolic comorbidities: systematic review and meta-analysis. Pediatr Dermatol. 2020;37:661–9.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics Declarations
In France and Italy, an ethics declaration is not necessary for retrospective data. We only required “non-opposition for participating to the study”.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of interest
V. Di Lernia: AbbVie and Novartis. J. Gottlieb: AbbVie, Celgene, Lilly, Novartis, Janssen Cilag, and UCB. N. Quiles-Tsimaratos: AbbVie, Celgene, Janssen Cilag, Leo Pharma, Lilly, Novartis, Pfizer, and UCB. H. Barthelemy: AbbVie, JanssenCilag, Leo Pharma, and Pfizer. I. Neri: Janssen Cilag, Sanofi, and Lilly. E. Mahé: AbbVie, Amgen, Celgene, Janssen Cilag, Leo Pharma, Lilly, and Novartis.
Ethics approval
We required the “non-opposition for participating to the study”.
Consent to participate
We required the “non-opposition for participating to the study”.
Consent for publication
We required the “non-opposition for participating to the study”.
Availability of data and materials
Data available on request to E. Mahé.
Code availability
Not applicable.
Author contributions
All authors: interpretation of data; revised critically the work, approved the version to be published. Vito Di Lernia, Anne-Claire Bursztejn, Juliette Mazereeuw-Hautier, Jérémy Gottlieb, Audrey Lasek, Hélène Aubert, Catherine Droitcourt, Cristina Bulai-Livideanu, Anna Belloni Fortina, Francesca Caroppo, Nathalie Quiles-Tsimaratos, Stéphanie Mallet, Hugues Barthélémy, Eve Puzenat, Danielle Bouilly-Auvray, Iria Neri,and Céline Phan: acquisition of data. Alain Beauchet and Raphaëlle Curmin: statistical analysis. J Zitouni and Emmanuel Mahé: conception, design, acquisition, part of analysis, creation of network, wrote the article.
Rights and permissions
About this article
Cite this article
Zitouni, J., Beauchet, A., Curmin, R. et al. Effectiveness and Safety of Adalimumab, Etanercept and Ustekinumab for Severe Psoriasis in Children Under 12 Years of Age: A French-Italian Daily Practice Cohort (BiPe Jr). Pediatr Drugs 24, 281–292 (2022). https://doi.org/10.1007/s40272-022-00501-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00501-6