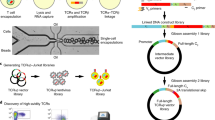
Abstract
It is commonly understood that T cells are activated via trans interactions between antigen-specific T-cell receptors (TCRs) and antigenic peptides presented on major histocompatibility complex (MHC) molecules on antigen-presenting cells. By analysing a large number of T cells at the single-cell level on a microwell array, we show that T-cell activation can occur via cis interactions (where TCRs on the T cell interact with the antigenic peptides presented on MHC class-I molecules on the same cell), and that such cis activation can be used to detect antigen-specific T cells and clone their TCR within 4 d. We used the detection-and-cloning system to clone a tumour-antigen-specific TCR from peripheral blood mononuclear cells of healthy donors. TCR cloning by leveraging the cis activation of T cells may facilitate the development of TCR-engineered T cells for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The cDNA sequences of the TCRs are available from the DNA Data Bank of Japan, under the set of accession codes LC663613–LC663622. The raw data generated during the study are available for research purposes from the corresponding author on reasonable request.
References
Held, W. & Mariuzza, R. A. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat. Rev. Immunol. 8, 269–278 (2008).
Garcia, K. C. et al. An αß T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science 274, 209–219 (1996).
Jin, A. et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat. Med. 15, 1088–1092 (2009).
Tokimitsu, Y. et al. Single lymphocyte analysis with a microwell array chip. Cytometry A 71, 1003–1010 (2007).
Kobayashi, E. et al. A new cloning and expression system yields and validates TCRs from blood lymphocytes of patients with cancer within 10 days. Nat. Med. 19, 1542–1546 (2013).
Pang, S. S. et al. The structural basis for autonomous dimerization of the pre-T-cell antigen receptor. Nature 467, 844–848 (2010).
Tawara, I. et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 130, 1985–1994 (2017).
Chapuis, A. G. et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 25, 1064–1072 (2019).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21, 914–921 (2015).
Clarke, S. R. et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78, 110–117 (2000).
Ober, B. T. et al. Affinity of thymic self-peptides for the TCR determines the selection of CD8(+) T lymphocytes in the thymus. Int. Immunol. 12, 1353–1363 (2000).
Betts, M. R. et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281, 65–78 (2003).
Zehn, D., Lee, S. Y. & Bevan, M. J. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
Fu, G. et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 504, 441–445 (2013).
Mareeva, T., Lebedeva, T., Anikeeva, N., Manser, T. & Sykulev, Y. Antibody specific for the peptide.major histocompatibility complex. Is it T cell receptor-like? J. Biol. Chem. 279, 44243–44249 (2004).
Linsley, P. S. & Ledbetter, J. A. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 11, 191–212 (1993).
Yang, J. et al. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proc. Natl Acad. Sci. USA 107, 4716–4721 (2010).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Santos, A. M. et al. Capturing resting T cells: the perils of PLL. Nat. Immunol. 19, 203–205 (2018).
Baniyash, M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat. Rev. Immunol. 4, 675–687 (2004).
Desombere, I. et al. The interferon gamma secretion assay: a reliable tool to study interferon gamma production at the single cell level. J. Immunol. Methods 286, 167–185 (2004).
Hu, Z. et al. A cloning and expression system to probe T-cell receptor specificity and assess functional avidity to neoantigens. Blood 132, 1911–1921 (2018).
Aleksic, M. et al. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur. J. Immunol. 42, 3174–3179 (2012).
Cheever, M. A. et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 15, 5323–5337 (2009).
Tsuboi, A. et al. Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol. Immunother. 51, 614–620 (2002).
Ge, Q. et al. Soluble peptide-MHC monomers cause activation of CD8+ T cells through transfer of the peptide to T cell MHC molecules. Proc. Natl Acad. Sci. USA 99, 13729–13734 (2002).
Pearse, B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc. Natl Acad. Sci. USA 73, 1255–1259 (1976).
Anderson, R. G. The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225 (1998).
Garcia, K. C. et al. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science 279, 1166–1172 (1998).
von Boehmer, H. et al. Control of T-cell development by the TCR alpha beta for antigen. Cold Spring Harb. Symp. Quant. Biol. 54, 111–118 (1989).
Persaud, S. P., Parker, C. R., Lo, W. L., Weber, K. S. & Allen, P. M. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat. Immunol. 15, 266–274 (2014).
Lyu, F. et al. A novel and simple method to produce large amounts of recombinant soluble peptide/major histocompatibility complex monomers for analysis of antigen-specific human T cell receptors. N. Biotechnol. 49, 169–177 (2019).
Cohen, C. J. et al. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J. Clin. Invest. 125, 3981–3991 (2015).
Tran, E. et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350, 1387–1390 (2015).
Freeman, J. D., Warren, R. L., Webb, J. R., Nelson, B. H. & Holt, R. A. Profiling the T-cell receptor beta-chain repertoire by massively parallel sequencing. Genome Res. 19, 1817–1824 (2009).
Robins, H. S. et al. Comprehensive assessment of T-cell receptor beta-chain diversity in αβ T cells. Blood 114, 4099–4107 (2009).
Wang, C. et al. High throughput sequencing reveals a complex pattern of dynamic interrelationships among human T cell subsets. Proc. Natl Acad. Sci. USA 107, 1518–1523 (2010).
Linnemann, C. et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat. Med. 19, 1534–1541 (2013).
Turchaninova, M. A. et al. Pairing of T-cell receptor chains via emulsion PCR. Eur. J. Immunol. 43, 2507–2515 (2013).
Han, A., Glanville, J., Hansmann, L. & Davis, M. M. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol. 32, 684–692 (2014).
Kondo, T. et al. Notch-mediated conversion of activated T cells into stem cell memory-like T cells for adoptive immunotherapy. Nat. Commun. 8, 15338 (2017).
Mo, F. et al. An engineered IL-2 partial agonist promotes CD8(+) T cell stemness. Nature 597, 544–548 (2021).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, eaba1786 (2021).
Ueno, T., Tomiyama, H., Fujiwara, M., Oka, S. & Takiguchi, M. Functionally impaired HIV-specific CD8 T cells show high affinity TCR-ligand interactions. J. Immunol. 173, 5451–5457 (2004).
Miyahara, Y. et al. Determination of cellularly processed HLA-A2402-restricted novel CTL epitopes derived from two cancer germ line genes, MAGE-A4 and SAGE. Clin. Cancer Res. 11, 5581–5589 (2005).
Hamana, H., Shitaoka, K., Kishi, H., Ozawa, T. & Muraguchi, A. A novel, rapid and efficient method of cloning functional antigen-specific T-cell receptors from single human and mouse T-cells. Biochem. Biophys. Res. Commun. 474, 709–714 (2016).
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 (2000).
Kinsella, T. M. & Nolan, G. P. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7, 1405–1413 (1996).
Shitaoka, K. et al. Identification of tumoricidal TCRs from tumor-infiltrating lymphocytes by single-cell analysis. Cancer Immunol. Res. 6, 378–388 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Acknowledgements
Melanoma-associated antigen (MAGEA4)-specific TCR (2–28) expression vectors were kindly provided by H. Shiku (Mie University). TCR sequence data of Wilms tumour 1 (WT1)-specific TCR (TAK1) were kindly provided by M. Yasukawa (Ehime University), PLAT-E cell line by T. Kitamura (University of Tokyo), human CD8-expressing TG40 cell line by T. Ueno (Kumamoto University) with permission from T. Saito (Riken), T2-A24 cell line by K. Kuzushima (Aichi Cancer Center Research Institute) and Phoenix-A cell line by G. Nolan (Stanford University). We thank T. Katagiri (University of Toyama) for helpful discussion. This research was supported by Practical Research for Innovative Cancer Control, Project for Cancer Research and Therapeutic Evolution (P-CREATE) from AMED under Grant Number JP17cm0106417 (E.K.) and JP20cm0106371 (E.K.), and JSPS KAKENHI grant numbers 18K19441 (H.K.), 16H06499 (H.K.) and 16H06500 (S.Y.), 21K18261 (H.K.), 21H02782 (E.K.) and 21H02966 (A.M.)
Author information
Authors and Affiliations
Contributions
E.K., H.K. and A.M. proposed the concept of cis interaction of TCR with pMHC molecules and of the T-ISAAC system. E.K., H.K. and A.J. designed the research studies. H.H. produced DNA constructs. T. Obata produced microwell-array chips. E.K., H.K., A.J., H.H., K.S., K.T. and T. Ozawa performed the research and analysed the data. S.K. and S.Y. built the structure model. A.J., H.K., E.K and A.M. discussed the results and implications. E.K., H.K. and A.M. wrote the paper. All authors read and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.K and A.M. are directors of SC World, Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and methods.
Rights and permissions
About this article
Cite this article
Kobayashi, E., Jin, A., Hamana, H. et al. Rapid cloning of antigen-specific T-cell receptors by leveraging the cis activation of T cells. Nat. Biomed. Eng 6, 806–818 (2022). https://doi.org/10.1038/s41551-022-00874-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00874-6
This article is cited by
-
Multimodal probing of T-cell recognition with hexapod heterostructures
Nature Methods (2024)
-
T-ISAAC: a novel TCR cloning system derived from T cells’ cis activation
Signal Transduction and Targeted Therapy (2022)