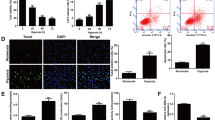
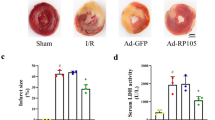
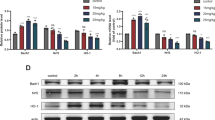
Abstract
Accumulating evidence suggests that autophagy dysfunction plays a critical role in myocardial ischemia/reperfusion (I/R) injury. However, the underling mechanism of malfunctional autophagy in the cardiomyocytes subjected to I/R has not been well defined. As a result, there is no effective therapeutic option by targeting autophagy to prevent myocardial I/R injury. Here, we used both an in vitro and an in vivo I/R model to monitor autophagic flux in the cardiomyocytes, by exposing neonatal rat ventricular myocytes to hypoxia/reoxygenation and by subjecting mice to I/R, respectively. We observed that the autophagic flux in the cardiomyocytes subjected to I/R was blocked in both in vitro and in vivo models. Down-regulating a lysosomal cationic channel, TRPML1, markedly restored the blocked myocardial autophagic flux induced by I/R, demonstrating that TRPML1 directly contributes to the blocked autophagic flux in the cardiomyocytes subjected to I/R. Mechanistically, TRPML1 is activated secondary to ROS elevation following ischemia/reperfusion, which in turn induces the release of lysosomal zinc into the cytosol and ultimately blocks the autophagic flux in cardiomyocytes, presumably by disrupting the fusion between autophagosomes and lysosomes. As a result, the inhibited myocardial autophagic flux induced by TRPML1 disrupted mitochondria turnover and resulted in mass accumulation of damaged mitochondria and further ROS release, which directly led to cardiomyocyte death. More importantly, pharmacological and genetic inhibition of TRPML1 channels greatly reduced infarct size and rescued heart function in mice subjected to I/R in vivo by restoring impaired myocardial autophagy. In summary, our study demonstrates that secondary to ROS elevation, activation of TRPML1 results in autophagy inhibition in the cardiomyocytes subjected to I/R, which directly leads to cardiomyocyte death by disrupting mitochondria turnover. Therefore, targeting TRPML1 represents a novel therapeutic strategy to protect against myocardial I/R injury.









Similar content being viewed by others
References
Aghaei M, Motallebnezhad M, Ghorghanlu S, Jabbari A, Enayati A, Rajaei M, Pourabouk M, Moradi A, Alizadeh AM, Khori V (2019) Targeting autophagy in cardiac ischemia/reperfusion injury: a novel therapeutic strategy. J Cell Physiol 234:16768–16778. https://doi.org/10.1002/jcp.28345
Aki T, Yamaguchi K, Fujimiya T, Mizukami Y (2003) Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene 22:8529–8535. https://doi.org/10.1038/sj.onc.1207197
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20:31–42. https://doi.org/10.1038/cdd.2012.81
Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM (2009) Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29:2570–2581. https://doi.org/10.1128/MCB.00166-09
Benischke AS, Vasanth S, Miyai T, Katikireddy KR, White T, Chen Y, Halilovic A, Price M, Price F Jr, Liton PB, Jurkunas UV (2017) Activation of mitophagy leads to decline in Mfn2 and loss of mitochondrial mass in Fuchs endothelial corneal dystrophy. Sci Rep 7:6656. https://doi.org/10.1038/s41598-017-06523-2
Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ (1997) The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243:240–246. https://doi.org/10.1111/j.1432-1033.1997.0240a.x
Borges JP, Lessa MA (2015) Mechanisms involved in exercise-induced cardioprotection: a systematic review. Arq Bras Cardiol 105:71–81. https://doi.org/10.5935/abc.20150024
Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femmino S, Garcia-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhauser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schluter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G (2018) Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 113:39. https://doi.org/10.1007/s00395-018-0696-8
Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Rande JL, Unterseeh T, Le Breton H, Beard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M (2015) Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 373:1021–1031. https://doi.org/10.1056/NEJMoa1505489
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3:461–491. https://doi.org/10.3233/JPD-130230
Ding WX, Yin XM (2012) Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393:547–564. https://doi.org/10.1515/hsz-2012-0119
Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H (2008) The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455:992–996. https://doi.org/10.1038/nature07311
Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK (2006) Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol 291:H2533-2540. https://doi.org/10.1152/ajpheart.00472.2006
Egan D, Kim J, Shaw RJ, Guan KL (2011) The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7:643–644. https://doi.org/10.4161/auto.7.6.15123
Fu H, Li X, Tan J (2018) NIPAAm-MMA nanoparticle-encapsulated visnagin ameliorates myocardial ischemia/reperfusion injury through the promotion of autophagy and the inhibition of apoptosis. Oncol Lett 15:4827–4836. https://doi.org/10.3892/ol.2018.7922
Gao Q, Zhao J, Fan Z, Bao J, Sun D, Li H, Sun C, Jiang X (2017) Cardioprotective effect of Danshensu against ischemic/reperfusion injury via c-Subunit of ATP synthase inhibition. Evid Based Complement Altern Med 2017:7986184. https://doi.org/10.1155/2017/7986184
Gedik N, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G, Kleinbongard P (2014) No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLoS One 9:e96567. https://doi.org/10.1371/journal.pone.0096567
Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol 6:524–551. https://doi.org/10.1016/j.redox.2015.08.020
Gustafsson AB, Gottlieb RA (2009) Autophagy in ischemic heart disease. Circ Res 104:150–158. https://doi.org/10.1161/CIRCRESAHA.108.187427
Hamacher-Brady A, Brady NR, Gottlieb RA (2006) Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281:29776–29787. https://doi.org/10.1074/jbc.M603783200
Hausenloy DJ, Yellon DM (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Investig 123:92–100. https://doi.org/10.1172/JCI62874
Heusch G (2020) Myocardial ischemia–reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z, Dai K, Wang C, Huang W (2015) Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. Eur J Pharmacol 762:1–10. https://doi.org/10.1016/j.ejphar.2015.05.028
Hughes WE, Beyer AM, Gutterman DD (2020) Vascular autophagy in health and disease. Basic Res Cardiol 115:41. https://doi.org/10.1007/s00395-020-0802-6
Kanamori H, Takemura G, Goto K, Maruyama R, Ono K, Nagao K, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Kawasaki M, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S (2011) Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol 300:H2261-2271. https://doi.org/10.1152/ajpheart.01056.2010
Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A (2005) TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem 280:43218–43223. https://doi.org/10.1074/jbc.M508210200
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, Abeliovich H, Abildgaard MH, Abudu YP, Acevedo-Arozena A, Adamopoulos IE, Adeli K, Adolph TE, Adornetto A, Aflaki E, Agam G, Agarwal A, Aggarwal BB, Agnello M, Agostinis P, Agrewala JN, Agrotis A, Aguilar PV, Ahmad ST, Ahmed ZM, Ahumada-Castro U, Aits S, Aizawa S, Akkoc Y, Akoumianaki T, Akpinar HA, Al-Abd AM, Al-Akra L, Al-Gharaibeh A, Alaoui-Jamali MA, Alberti S, Alcocer-Gomez E, Alessandri C, Ali M, Alim Al-Bari MA, Aliwaini S, Alizadeh J, Almacellas E, Almasan A, Alonso A, Alonso GD, Altan-Bonnet N, Altieri DC, Alvarez EMC, Alves S, Alves da Costa C, Alzaharna MM, Amadio M, Amantini C, Amaral C, Ambrosio S, Amer AO, Ammanathan V, An Z, Andersen SU, Andrabi SA, Andrade-Silva M, Andres AM, Angelini S, Ann D, Anozie UC, Ansari MY, Antas P, Antebi A, Anton Z, Anwar T, Apetoh L, Apostolova N, Araki T, Araki Y, Arasaki K, Araujo WL, Araya J, Arden C, Arevalo MA, Arguelles S, Arias E, Arikkath J, Arimoto H, Ariosa AR, Armstrong-James D, Arnaune-Pelloquin L, Aroca A, Arroyo DS, Arsov I, Artero R, Asaro DML, Aschner M, Ashrafizadeh M, Ashur-Fabian O, Atanasov AG, Au AK, Auberger P, Auner HW, Aurelian L et al (2021) Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 17:1–382. https://doi.org/10.1080/15548627.2020.1797280
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524:309–314. https://doi.org/10.1038/nature14893
Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314:H812–H838. https://doi.org/10.1152/ajpheart.00335.2017
Ma S, Wang Y, Chen Y, Cao F (2015) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim Biophys Acta 1852:271–276. https://doi.org/10.1016/j.bbadis.2014.05.010
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Diwan A (2012) Autophagy is impaired in cardiac ischemia–reperfusion injury. Autophagy 8:1394–1396. https://doi.org/10.4161/auto.21036
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125:3170–3181. https://doi.org/10.1161/CIRCULATIONAHA.111.041814
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922. https://doi.org/10.1161/01.RES.0000261924.76669.36
Miedel MT, Rbaibi Y, Guerriero CJ, Colletti G, Weixel KM, Weisz OA, Kiselyov K (2008) Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. J Exp Med 205:1477–1490. https://doi.org/10.1084/jem.20072194
Mills M, Yang N, Weinberger R, Vander Woude DL, Beggs AH, Easteal S, North K (2001) Differential expression of the actin-binding proteins, alpha-actinin-2 and -3, in different species: implications for the evolution of functional redundancy. Hum Mol Genet 10:1335–1346. https://doi.org/10.1093/hmg/10.13.1335
Mo Y, Tang L, Ma Y, Wu S (2016) Pramipexole pretreatment attenuates myocardial ischemia/reperfusion injury through upregulation of autophagy. Biochem Biophys Res Commun 473:1119–1124. https://doi.org/10.1016/j.bbrc.2016.04.026
Moris D, Spartalis M, Spartalis E, Karachaliou GS, Karaolanis GI, Tsourouflis G, Tsilimigras DI, Tzatzaki E, Theocharis S (2017) The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann Transl Med 5:326. https://doi.org/10.21037/atm.2017.06.27
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S (2015) Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131:e29-322. https://doi.org/10.1161/CIR.0000000000000152
Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K (2009) The role of autophagy in the heart. Cell Death Differ 16:31–38. https://doi.org/10.1038/cdd.2008.163
Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JP (2012) Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia–reperfusion in vivo. Basic Res Cardiol 107:270. https://doi.org/10.1007/s00395-012-0270-8
Osorio L, Gijsbers R, Oliveras-Salva M, Michiels A, Debyser Z, Van den Haute C, Baekelandt V (2014) Viral vectors expressing a single microRNA-based short-hairpin RNA result in potent gene silencing in vitro and in vivo. J Biotechnol 169:71–81. https://doi.org/10.1016/j.jbiotec.2013.11.004
Piper HM, Garcia-Dorado D, Ovize M (1998) A fresh look at reperfusion injury. Cardiovasc Res 38:291–300. https://doi.org/10.1016/s0008-6363(98)00033-9
Przyklenk K, Dong Y, Undyala VV, Whittaker P (2012) Autophagy as a therapeutic target for ischemia/reperfusion injury? Concepts, controversies, and challenges. Cardiovasc Res 94:197–205. https://doi.org/10.1093/cvr/cvr358
Qi J, Xing Y, Liu Y, Wang MM, Wei X, Sui Z, Ding L, Zhang Y, Lu C, Fei YH, Liu N, Chen R, Wu M, Wang L, Zhong Z, Wang T, Liu Y, Wang Y, Liu J, Xu H, Guo F, Wang W (2021) MCOLN1/TRPML1 finely controls oncogenic autophagy in cancer by mediating zinc influx. Autophagy. https://doi.org/10.1080/15548627.2021.1917132
Sahoo N, Gu M, Zhang X, Raval N, Yang J, Bekier M, Calvo R, Patnaik S, Wang W, King G, Samie M, Gao Q, Sahoo S, Sundaresan S, Keeley TM, Wang Y, Marugan J, Ferrer M, Samuelson LC, Merchant JL, Xu H (2017) Gastric acid secretion from parietal cells is mediated by a Ca(2+) efflux channel in the tubulovesicle. Dev Cell 41(262–273):e266. https://doi.org/10.1016/j.devcel.2017.04.003
Sivandzade F, Bhalerao A, Cucullo L (2019) Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bioprotocol. https://doi.org/10.21769/BioProtoc.3128
Sun D, Melegari M, Sridhar S, Rogler CE, Zhu L (2006) Multi-miRNA hairpin method that improves gene knockdown efficiency and provides linked multi-gene knockdown. Biotechniques 41:59–63. https://doi.org/10.2144/000112203
Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J (2007) AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy 3:405–407. https://doi.org/10.4161/auto.4281
Thielmann M, Sharma V, Al-Attar N, Bulluck H, Bisleri G, Bunge JJH, Czerny M, Ferdinandy P, Frey UH, Heusch G, Holfeld J, Kleinbongard P, Kunst G, Lang I, Lentini S, Madonna R, Meybohm P, Muneretto C, Obadia JF, Perrino C, Prunier F, Sluijter JPG, Van Laake LW, Sousa-Uva M, Hausenloy DJ (2017) ESC Joint Working Groups on Cardiovascular Surgery and the Cellular Biology of the Heart Position Paper: perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur Heart J 38:2392–2407. https://doi.org/10.1093/eurheartj/ehx383
Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A (2006) Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol 40:846–852. https://doi.org/10.1016/j.yjmcc.2006.03.428
Walters AM, Porter GA Jr, Brookes PS (2012) Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res 111:1222–1236. https://doi.org/10.1161/CIRCRESAHA.112.265660
Wang F, He Q, Gao Z, Redington AN (2021) Atg5 knockdown induces age-dependent cardiomyopathy which can be rescued by repeated remote ischemic conditioning. Basic Res Cardiol 116:47. https://doi.org/10.1007/s00395-021-00888-2
Wang W, Gao Q, Yang M, Zhang X, Yu L, Lawas M, Li X, Bryant-Genevier M, Southall NT, Marugan J, Ferrer M, Xu H (2015) Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc Natl Acad Sci USA 112:E1373-1381. https://doi.org/10.1073/pnas.1419669112
Wang W, Zhang X, Gao Q, Xu H (2014) TRPML1: an ion channel in the lysosome. Handb Exp Pharmacol 222:631–645. https://doi.org/10.1007/978-3-642-54215-2_24
Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA (2014) Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 129:1139–1151. https://doi.org/10.1161/CIRCULATIONAHA.113.002416
Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23:33–42. https://doi.org/10.1247/csf.23.33
Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF (2005) Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA 102:13807–13812. https://doi.org/10.1073/pnas.0506843102
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135. https://doi.org/10.1056/NEJMra071667
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9–14. https://doi.org/10.1038/nrm3028
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283:10892–10903. https://doi.org/10.1074/jbc.M800102200
Zhang H, Yang N, He H, Chai J, Cheng X, Zhao H, Zhou D, Teng T, Kong X, Yang Q, Xu Z (2021) The zinc transporter ZIP7 (Slc39a7) controls myocardial reperfusion injury by regulating mitophagy. Basic Res Cardiol 116:54. https://doi.org/10.1007/s00395-021-00894-4
Zhang L, Wang H, Zhao Y, Wang J, Dubielecka PM, Zhuang S, Qin G, Chin YE, Kao RL, Zhao TC (2018) Myocyte-specific overexpressing HDAC4 promotes myocardial ischemia/reperfusion injury. Mol Med 24:37. https://doi.org/10.1186/s10020-018-0037-2
Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, Delling M, Marugan J, Ferrer M, Xu H (2016) MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun 7:12109. https://doi.org/10.1038/ncomms12109
Zhang YJ, Zhang M, Zhao X, Shi K, Ye M, Tian J, Guan S, Ying W, Qu X (2020) NAD(+) administration decreases microvascular damage following cardiac ischemia/reperfusion by restoring autophagic flux. Basic Res Cardiol 115:57. https://doi.org/10.1007/s00395-020-0817-z
Zhao Y, Pan R, Li S, Luo Y, Yan F, Yin J, Qi Z, Yan Y, Ji X, Liu KJ (2014) Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke 45:1139–1147. https://doi.org/10.1161/STROKEAHA.113.004296
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC) Grants (81772559 to W. W; 82101314 to Y. X; 81600967 to C. L; 81971212 to F. G), NSF grants of the Jiangsu Province (BK20170262 to W. W), Key University Science Research Project of Jiangsu Province (20KJA310001 to W.W), Jiangsu Specially-Appointed Professor award to W.W, Jiangsu Province Innovative and Entrepreneurial Talent program to W.W and Jiangsu Province Innovative and Entrepreneurial Team program to W.W. Natural Science Foundation of Liaoning Province (2021-MS-161 to M.M. W.). We are grateful to Dr. Hailey Jansen (Libin Cardiovascular Institute, University of Calgary, Canada) for critical reading through the manuscript and appreciate the encouragement and helpful comments from other members of the Wang laboratory.
Author information
Authors and Affiliations
Contributions
WW and JQ conceived of the presented idea. YX, ZS, LY, MW, XW, QL, NL, CL, RC, MW, YW, YHZ, FG, and JQ performed experiments and contributed to the interpretation of the results. WW, JQ, FG and JLC supervised the findings of this work. WW and JQ wrote the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors disclose no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xing, Y., Sui, Z., Liu, Y. et al. Blunting TRPML1 channels protects myocardial ischemia/reperfusion injury by restoring impaired cardiomyocyte autophagy. Basic Res Cardiol 117, 20 (2022). https://doi.org/10.1007/s00395-022-00930-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-022-00930-x