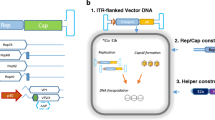
Abstract
Recombinant adeno-associated virus (rAAV) is one of the most widely used viral vectors that has been used for gene therapy to treat a variety of human diseases. As a newly developed product type with growing demand, understanding process performance is critical. In this study, flowsheet modeling is used to design a rAAV drug manufacturing process operating in batch and continuous mode. The designed plant has the capability to reach an annual production rate close to 2.7 × 1019 vg/year. Economic analysis is used to analyze the cost and provide a breakdown into specific categories and unit operations. The Benzonase® nuclease used in the primary clarification contributes the highest amount among the overall costs for both batch and continuous processes. For batch operation, the most cost-effective production rate is 2.6 × 1019 vg/year which is obtained by applying process debottlenecking method. To understand the process flexibility, different manufacturing scales are compared for batch and continuous operation. The analysis illustrated that the continuous operation becomes advantageous for production above 0.5 × 1019 vg/year whereas batch operation is more cost-effective to supply AAV products that are less than 0.5 × 1019 vg/year.









Similar content being viewed by others
References
Ma C-C, Wang Z-L, Xu T, He Z-Y, Wei Y-Q. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40:107502. https://doi.org/10.1016/j.biotechadv.2019.107502.
MarketsandMarkets. Gene Therapy Market by Vectors [Non-viral (Oligonucleotides), Viral (retroviral (gammaretroviral, lentiviral)), adeno-associated], indication (Cancer, Neurological Diseases), delivery method (in vivo, ex vivo), region - global forecast to 2024. 2018 https://www.marketsandmarkets.com/Market-Reports/gene-therapy-market-122857962.html?gclid=Cj0KCQjw0caCBhCIARIsAGAfuMzdbr5WyZB9ibIkLu-nQxsfQ5p58KeZzRfnDMv8TQlJmcTCb5i4V00aAt6lEALw_wcB. Accessed 17 Mar 2021.
Gonçalves GAR, Paiva RDMA. Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo). 2017;15(3):369–75. https://doi.org/10.1590/S1679-45082017RB4024.
Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20(5):e3015. https://doi.org/10.1002/jgm.3015.
Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here?. Annu Rev Virol. 2019;6(1):601–21. https://doi.org/10.1146/annurev-virology-092818-015530.
Smith DC. AAV vector manufacturing – challenges & opportunities in the manufacturing of aav vectors used in the delivery of gene therapy treatments. Drug Development and Delivery. 2017. https://drug-dev.com/aav-vector-manufacturing-challenges-opportunities-in-the-manufacturing-of-aav-vectors-used-in-the-delivery-of-gene-therapy-treatments/
Clément N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol Ther - Methods Clin Dev. 2016;3:16002. https://doi.org/10.1038/mtm.2016.2.
Grieger JC, Soltys SM, Samulski RJ. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol Ther. 2016;24(2):287–97. https://doi.org/10.1038/mt.2015.187.
Hernandez Bort JA. Challenges in the downstream process of gene therapy products. Am Pharm Rev. 2019;22:25.
Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358–78. https://doi.org/10.1038/s41573-019-0012-9.
Merten O-W. AAV vector production: state of the art developments and remaining challenges. Cell Gene Ther Insights. 2016;2(5):521–51.
Qu W, Wang M, Wu Y, Xu R. Scalable downstream strategies for purification of recombinant adeno- associated virus vectors in light of the properties. Curr Pharm Biotechnol. 2015;16(8):684–95. https://doi.org/10.2174/1389201016666150505122228.
Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther-Methods Clin Dev. 2018;8:87–104.
Cameau E, Pedregal A, Glover C. Cost modelling comparison of static, suspension and fixed bed bioreactors to manufacture commercial gene therapy products. 2019;30(11):A41–A42. https://www.pall.com/en/biotech/posters-presentations/cost-modelling-comparison-static-suspension-fixed-bed-bioreactors-manufacture-commercial-gene-therapy-products.html. Accessed 3 April 2022.
Cytiva. Process economic simulation for scalable production of adenovirus. 2020. https://cdn.cytivalifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=27022. Accessed 18 Mar 2021.
Henry O, Dormond E, Perrier M, Kamen A. Insights into adenoviral vector production kinetics in acoustic filter-based perfusion cultures. Biotechnol Bioeng. 2004;86(7):765–74. https://doi.org/10.1002/bit.20074.
Silva RJS, Mendes JP, Carrondo MJT, Marques PM, Peixoto C. Continuous chromatography purification of virus-based biopharmaceuticals: a shortcut design method. Methods Mol Biol (Clifton, NJ). 2020;2095:367–84. https://doi.org/10.1007/978-1-0716-0191-4_21.
Silva RJS, Mota J, Peixoto C, Alves P, Carrondo MJ. Improving the downstream processing of vaccine and gene therapy vectors with continuous chromatography. Pharm Bioprocess. 2015;3:489–505.
Gränicher G, Coronel J, Trampler F, Jordan I, Genzel Y, Reichl U. Performance of an acoustic settler versus a hollow fiber–based ATF technology for influenza virus production in perfusion. Appl Microbiol Biotechnol. 2020;104(11):4877–88. https://doi.org/10.1007/s00253-020-10596-x.
Ayuso E, Mingozzi F, Bosch F. Production, purification and characterization of adeno-associated vectors. Curr Gene Ther. 2010;10(6):423–36. https://doi.org/10.2174/156652310793797685.
Growing pains for gene therapy manufacturing Vigene Biosciences, Advertisement feature https://www.nature.com/articles/d42473-018-00016-0. 2021. Accessed 4 April 2022.
Dias Florencio G, Precigout G, Beley C, Buclez PO, Garcia L, Benchaouir R. Simple downstream process based on detergent treatment improves yield and in vivo transduction efficacy of adeno-associated virus vectors. Mol Ther Methods Clin Dev. 2015;2:15024. https://doi.org/10.1038/mtm.2015.24.
Sandoval IM, Kuhn NM, Manfredsson FP. Multimodal production of adeno-associated virus. Methods Mol Biol. 2019;1937:101–24. https://doi.org/10.1007/978-1-4939-9065-8_6.
Besnard L, Fabre V, Fettig M, Gousseinov E, Kawakami Y, Laroudie N, et al. Clarification of vaccines: an overview of filter based technology trends and best practices. Biotechnol Adv. 2016;34(1):1–13. https://doi.org/10.1016/j.biotechadv.2015.11.005.
Durocher Y, Pham PL, St-Laurent G, Jacob D, Cass B, Chahal P, et al. Scalable serum-free production of recombinant adeno-associated virus type 2 by transfection of 293 suspension cells. J Virol Methods. 2007;144(1–2):32–40. https://doi.org/10.1016/j.jviromet.2007.03.014.
Moleirinho MG, Rosa S, Carrondo MJ, Silva RJ, Hagner-McWhirter Å, Ahlén G, et al. Clinical-grade oncolytic adenovirus purification using polysorbate 20 as an alternative for cell Lysis. Curr Gene Ther. 2018;18(6):366–74.
Wright JF. Product-related impurities in clinical-grade recombinant AAV vectors: characterization and risk assessment. Biomedicines. 2014. https://doi.org/10.3390/biomedicines2010080.
Thorne BA, Takeya RK, Peluso RW. Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Hum Gene Ther. 2009;20(7):707–14. https://doi.org/10.1089/hum.2009.070.
Toueille M, Attebi E, Dejoint L, Cartigny J, Rasle C, Potier S, et al. Development of purification steps for several AAV serotypes using POROS™ CaptureSelect™ AAVX affinity chromatography. Cell Gene Ther Insights. 2018;4(7):9.
Pall. A different approach to adeno-associated virus (AAV) clarification. 2021. https://www.pall.com/en/biotech/blog/different-approach-aav-virus-clarification.html. Accessed 19 Mar 2022.
Pharma's Almanac. Thomas Parker YC, Claire Scanlan, Elina Gousseinov. Robust harvest clarification for adeno-associated viral vectors via depth filtration. 2020. https://www.pharmasalmanac.com/articles/robust-harvest-clarification-for-adeno-associated-viral-vectors-via-depth-filtration. Accessed 19 Mar 2022.
Millipore. Clarification Portfolio Guide Single- and multi-use products for the successful development and implementation of robust clarification processes. 2022. Accessed 4 April 2022.
Gutiérrez-Granados S, Gòdia F, Cervera L. Continuous manufacturing of viral particles. Curr Opin Chem Eng. 2021;22:107–14. https://doi.org/10.1016/j.coche.2018.09.009.
Escandell JM, Pais DAM, Carvalho SB, Vincent K, Gomes-Alves P, Alves PM. Leveraging rAAV bioprocess understanding and next generation bioanalytics development. Curr Opin Biotechnol. 2022;74:271–7. https://doi.org/10.1016/j.copbio.2021.12.009.
Coronel J, Patil A, Al-Dali A, Braβ T, Faust N, Wissing S. Efficient production of rAAV in a perfusion bioreactor using an ELEVECTA® stable producer cell line. Genet Eng Biotechnol News. 2021;41(S2):S23–S23. https://doi.org/10.1089/gen.41.S2.07.
Funding
This work received technical support from Eli Lilly and Company and financial support from U.S. Food and Drug Administration, grant number DHHS-FDA-R01FD006588, and NSF, grant number 1933584.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to Participate
Informed consent is not applicable to this article.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, O., Tao, Y., Qadan, M. et al. Process Design and Comparison for Batch and Continuous Manufacturing of Recombinant Adeno-Associated Virus. J Pharm Innov 18, 275–286 (2023). https://doi.org/10.1007/s12247-022-09645-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09645-x