Abstract
Background
Biosimilars have been adopted by clinicians more slowly than anticipated in the post-marketing phase.
Objectives
We aimed to reveal the perceptions and attitudes of pediatric rheumatologists towards biosimilars and the obstacles to biosimilar therapy.
Methods
A web-based survey designed to determine the knowledge, experience, and perceptions of pediatric rheumatologists about biosimilars was electronically mailed to the participants between April and August 2021. Responses were collected anonymously and subsequently analyzed.
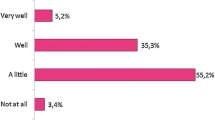
Results
A total of 114 pediatric rheumatologists including fellows (32.4%), specialists (29.8%), and seniors (37.7%) responded to the questionnaire. According to the data, 75 (65.8%) physicians had already prescribed at least one biosimilar. The vast majority of participants were aware of the potential cost savings of biosimilars (84, 73.3%). Participants who felt insufficiently informed were 41.8%, 67.6%, and 83.7% among seniors, specialists, and fellows, respectively. In pediatric rheumatology, the scarcity of clinical trials and real-life data (64%) and inadequate information about tolerance to the biosimilars and related side effects in children (49.1%) were the most common barriers expressed by prescribers. Nearly half (45%) of the pediatric rheumatologists preferred to prescribe biosimilars in the treatment of biologic-naive cases. However, most (93%) were reluctant to switch a reference molecule to a biosimilar while the patient was doing well under the originator medicine.
Conclusions
This survey provided insights into the concerns about prescribing biosimilars among pediatric rheumatologists. In the field of pediatric rheumatology, further education about biosimilars and real-life experiences is required to better inform about treatment options in children.





Similar content being viewed by others
References
Lahaye C, Tatar Z, Dubost JJ, Soubrier M. Overview of biologic treatments in the elderly. Jt Bone Spine. 2015;82(3):154–60.
Strand V, Goncalves J, Isaacs JD. Immunogenicity of biologic agents in rheumatology. Nat Rev Rheumatol. 2021;17(2):81–97.
Cimaz R, Maioli G, Calabrese G. Current and emerging biologics for the treatment of juvenile idiopathic arthritis. Expert Opin Biol Ther. 2020;20(7):725–40.
Cabrera N, Lega JC, Kassai B, Wouters C, Kondi A, Cannizzaro E, et al. Safety of biological agents in paediatric rheumatic diseases: a real-life multicenter retrospective study using the JIRcohorte database. Jt Bone Spine. 2019;86(3):343–50.
Kuemmerle-Deschner JB, Gautam R, George AT, Raza S, Lomax KG, Hur P. A systematic literature review of efficacy, effectiveness and safety of biologic therapies for treatment of familial Mediterranean fever. Rheumatology (Oxf). 2020;59(10):2711–24.
Top selling biologics market, 2021–2030: focus on product landscape assessment, ongoing clinical trials, promotional content analysis, other life cycle management strategies, competition from biosimilars, annual treatment cost comparison, sales evolution and future opportunity. 2021. https://www.businesswire.com/news/home/20210803005814/en/Global-Top-Selling-Biologics-Market-Report-2021-2030---ResearchAndMarkets.com.
Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, et al. Phage display derived monoclonal antibodies: from bench to bedside. Front Immunol. 2020;11:1986.
US Food and Drug Administration. Guidances (drugs): biosimilars. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm290967.htm. Accessed 28 Nov 2019.
Moorkens E, Godman B, Huys I, Hoxha I, Malaj A, Keuerleber S, et al. The expiry of Humira® market exclusivity and the entry of adalimumab biosimilars in Europe: an overview of pricing and national policy measures. Front Pharmacol. 2020;11:591134.
Leonard E. Factors affecting healthcare provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102–12. https://doi.org/10.17615/cky7-df88.
O’Callaghan J, Bermingham M, Leonard M, Hallinan F, Morris JM, Moore U, et al. Assessing awareness and attitudes of healthcare professionals on the use of biosimilar medicines: a survey of physicians and pharmacists in Ireland. Regul Toxicol Pharmacol. 2017;88:252–61.
Gibofsky A, McCabe D. US rheumatologists’ beliefs and knowledge about biosimilars: a survey. Rheumatology (Oxf). 2021;60(2):896–901.
Cohen H, Beydoun D, Chien D, Lessor T, McCabe D, Muenzberg M, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160–72.
Beck M, Michel B, Rybarczyk-Vigouret MC, Levêque D, Sordet C, Sibilia J, et al. Rheumatologists’ perceptions of biosimilar medicines prescription: findings from a French web-based survey. BioDrugs. 2016;30(6):585–92.
Reilly MS. Biosimilar substitution: European prescriber perspectives. Ann Oncol. 2019;30. https://www.annalsofoncology.org/article/S0923-7534(19)59837-4/pdf.
Ismailov RM, Khasanova ZD. Biosimilar knowledge among oncology/hematology team members in Colorado, USA: an educational initiative and follow-up survey. BioDrugs. 2018;32(5):499–506.
Leonard E, Wascovich M, Oskouei S, Gurz P, Carpenter D. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102–12.
Maccora I, Lombardi N, Crescioli G, Bettiol A, Bonaiuti R, Pagnini I, et al. OBSIDIAn: real world evidence of Originator to BioSImilar Drug switch in juvenile idiopathic arthritis. Rheumatology (Oxf). 2021. https://doi.org/10.1093/rheumatology/keab572.
Demirkan FG, Ulu K, Öztürk K, Karadağ ŞG, Özdel S, Sönmez HE, et al. Toward the integration of biosimilars into pediatric rheumatology: adalimumab ABP 501 experience of PeRA research group. Expert Opin Biol Ther. 2022;22(2):197–202.
Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36(10):3179–87.
Pasina L, Casadei G, Nobili A. A survey among hospital specialists and pharmacists about biosimilars. Eur J Intern Med. 2016;35:e31–3.
Cassar K, Zammit Dimech D, Grech L, Balzan D, Cutajar A, Cassar PJ. SAT0637-HPR biosimilars: the perception amongst Maltese clinicians. Ann Rheum Dis. 2016;75(Suppl. 2):1294.
Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463–78.
US Food and Drug Administration. Guidance for industry: immunogenicity assessment for therapeutic protein products. https://www.fda.gov/media/85017/download.
Hoven Avd. Biosimilar medicines: practical EU experience and perspectives. Paper presented at the AAM Biosimilars Council Conference; 2017; Washington, DC.
Barsell A, Rengifo-Pardo M, Ehrlich A. A survey assessment of US dermatologists’ perception of biosimilars. J Drugs Dermatol. 2017;16(6):612–5.
Adé A, Bourdon O, Bussières JF. A survey of pharmacists’ knowledge and views of biosimilars in Quebec and France. Ann Pharm Fr. 2017;75(4):267–75.
Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence. 2017;11:519–30.
Sullivan E, Piercy J, Waller J, Black CM, Kachroo S. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One. 2017;12(4):e0175826.
Narayanan S, Nag S. Likelihood of use and perception towards biosimilars in rheumatoid arthritis: a global survey of rheumatologists. Clin Exp Rheumatol. 2016;34(1 Suppl. 95):S9-11.
van Overbeeke E, De Beleyr B, de Hoon J, Westhovens R, Huys I. Perception of originator biologics and biosimilars: a survey among Belgian rheumatoid arthritis patients and rheumatologists. BioDrugs. 2017;31(5):447–59.
Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol. 2019;10:1372.
Pouillon L, Socha M, Demore B, Thilly N, Abitbol V, Danese S, et al. The nocebo effect: a clinical challenge in the era of biosimilars. Expert Rev Clin Immunol. 2018;14(9):739–49.
Brower CK, editor. Too long and too boring: the effects of survey length and interest on careless responding. 2018. Wright State University, Ohio.
Meade AW, Craig SB. Identifying careless responses in survey data. Psychol Methods. 2012;17(3):437–55.
Acknowledgements
We thank all the pediatric rheumatologists who participated in the study worldwide. We also thank Nicola Ruperto and PRINTO (Paediatric Rheumatology INternational Trials Organisation) and the PReS (European Society for Pediatric Rheumatology)-EMERGE (Emerging Rheumatologists and Researchers) group for disseminating the survey. All persons and members of the groups named in the Acknowledgments section have given us written permission to be named in the article.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Istanbul University (no. 21.05.2021-204228).
Consent to participate
Each of the respondents filled out the questionnaire voluntarily.
Consent for publication
No personal or confidential data were collected.
Availability of data and material
The data underlying this article will be shared on reasonable request to the corresponding author.
Code availability
Not applicable.
Authors’ contributions
All authors whose names appear on the submission: (1) made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; (2) drafted the work or revised it critically for important intellectual content; (3) approved the version to be published; and (4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the study conception and design. Methodology: Nuray Aktay Ayaz. Formal analysis and investigation: Fatma Gül Demirkan. Writing, original draft preparation: Fatma Gül Demirkan, Hafize Emine Sönmez, Özlem Akgün. Writing, review, and editing: Lovro Lamot, Betül Sözeri.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Demirkan, F.G., Sönmez, H.E., Lamot, L. et al. Embracing Change: An International Survey Study on the Beliefs and Attitudes of Pediatric Rheumatologists Towards Biosimilars. BioDrugs 36, 421–430 (2022). https://doi.org/10.1007/s40259-022-00526-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00526-w