Abstract
Living in a social group increases the risks of parasitism, especially in highly-related groups. In homogenous groups, with no reproductive division of labour, the impact of parasitism is unlikely to vary with host identity. Many social systems, however, do exhibit division of reproductive labour, most famously in social insects with their reproductive queens and generally infertile workers. In such systems, the impact of parasitism will differ for each group. Consequently, we predict that susceptibility to parasites will vary to reflect such differential impact. We tested this prediction using a trypanosome-bumble bee system, where Crithidia bombi infects both gynes and workers of Bombus terrestris. We studied both susceptibility to the parasite and relevant measures of the immune function. As predicted, gynes were significantly less susceptible to the parasite than workers, but while gynes and workers expressed different immune profiles, how these link to differential susceptibility remains unclear. In conclusion, our results suggest that differential selection pressures exerted by parasites may produce multiple phenotypes from a single genotype in order to maximise fitness in a social group context.
Significance statement
Social insect colonies dominate terrestrial ecology, and as such are targets for parasites. How they defend themselves against such threats is a key question. Here, we show that bumble bee gynes — the reproductive individuals that overwinter and found colonies in this annual social system — are more resistant to a parasite that disproportionately affects reproductive fitness than their sister workers. Differential patterns of susceptibility may help to explain the success of these social insects.
Similar content being viewed by others
Introduction
Many animals live in social groups. While advantageous (Krause and Ruxton 2002), living in a social group also imposes a range of costs on group members (Hamilton 1987; Krause and Ruxton 2002). One major cost of social life is an increased exposure to, and pressure from parasites (Anderson and May 1981; Côté and Poulin 1995). In social groups where there is no reproductive division of labour, parasitism is likely to have a similar impact on host fitness irrespective of which individual within a sex becomes infected. In contrast, in social groups with such division of labour (e.g. Wilson 1971; Jarvis 1981; Duffy 1996), parasite impact on the fitness of group members will depend upon which individuals become infected.
Social insects have crossed one of the major transitions in evolution (Maynard Smith and Szathmáry 1995) and represent one of the most advanced and complex social systems. Their social groups are fundamentally based on a division of labour between highly related reproductive and non-reproductive individuals, with the latter maintaining the colony through foraging, brood care and nest maintenance (Wilson 1971). Consequently, the hymenopteran social insect colonies produce two kinds of female offspring — workers (somatic growth) and sexuals, or gynes (the reproductive line, or female offspring destined to become queen) — from the same underlying genotype. Colony fitness depends ultimately on the success of new gynes, which, like long-lived queens in extant colonies, require greater protection from parasitism (Cremer et al. 2007) than do replaceable, short-lived workers (Keller and Genoud 1997). Such protection may be achieved through mate choice (e.g. Rosengaus and Traniello 1993), social behaviour (e.g. Cremer et al. 2007), immunity (e.g. Schmid et al. 2008), morphology (e.g. Hughes et al. 2009) or even diet (e.g. Giacomini et al. 2018; LoCascio et al. 2019). While previous studies suggest differential susceptibility to natural parasites in gynes/queens and workers (Fowler et al. 2020; Ulrich et al. 2011), and an increasing numbers of studies have examined mechanisms through which such differential protection may arise (see above), we know of no studies where differential susceptibility to natural parasites has been experimentally associated with caste differences in immunity.
Here, we use the trypanosome-bumble bee model system (Schmid-Hempel 2001) to test whether differential susceptibility to the parasite exists across female reproductive castes. Bumble bees (Bombus spp.) are annual, eusocial insects. Singly-mated mother queens (Schmid-Hempel and Schmid-Hempel 2000) produce workers and daughter queens that share, on average, 75% of their genes, irrespectively of caste differences. Nevertheless, workers and queens exhibit important life history differences, which probably result from both phenotypic variation and differential gene expression patterns as in honey bees (Sadd et al. 2015; The Honeybee Genome Sequencing Consortium 2006). Bumble bee queens emerge from hibernation in the spring and found colonies that grow in worker number until the production of sexuals (gynes and males). These sexuals leave the nest, mate and then the newly-mated queens (as the gynes have now become) enter hibernation. However, while the queens remain in the nest laying eggs, their female worker offspring work inside and outside of the colony, caring and cleaning the nest or foraging for nectar and pollen (Alford 1975). Crithidia bombi, a non-invasive gut trypanosome, is a highly prevalent parasite of bumble bees in Europe (e.g. Shykoff and Schmid-Hempel 1991a; but see Cordes et al. 2012 for different patterns in North America). C. bombi infection delays ovarian development and timing of oviposition in workers (Shykoff and Schmid-Hempel 1991b) and infected workers forage less efficiently (Otterstatter et al. 2005). Under starvation conditions, worker mortality rate increases by 50% (Brown et al. 2000). Infection of newly-mated queens results in a 40% fitness loss (measured as the number of reproductive offspring — males and gynes — produced) over the colony cycle (Brown et al. 2003a; Yourth et al. 2008) and reduces queen hibernation survival (Fauser et al. 2017). Bumble bee colonies are highly buffered against loss of workers (Schmid-Hempel and Heeb 1991). Consequently, while worker infection will have only minor effects on colony fitness (Shykoff and Schmid-Hempel 1991b), queen infection will have a dramatic impact, and we would expect to see differential susceptibility across the two castes. Correlational evidence for such differential susceptibility comes from field studies, where spring queens have lower prevalence of the trypanosome than workers from the previous summer (Shykoff and Schmid-Hempel 1991a), and laboratory studies found that infected colonies had a lower proportion of infected gynes than would be expected based on worker infection levels (Ulrich et al. 2011).
One mechanism for variation in susceptibility across queens and workers may be caste-specific expression of the immune system, as has been shown in honey bees and ants (Koch et al. 2013; Schmid et al. 2008). In its natural bumble bee hosts, C. bombi has been shown to elicit the constitutive and non-specific branch of the immune system, including the pro-phenoloxidase (proPO) cascade (Brown et al. 2003b; Otterstatter and Thomson 2006). The proPO cascade has previously been demonstrated to provide protection against invasive trypanosomes (Nigam et al. 1997). More recently, infection has been shown to elicit expression of antimicrobial peptides (e.g. Barribeau et al. 2014), and inhibition of peptide expression has demonstrated their role in preventing successful infection (Deshwal and Mallon 2014).
Here, we use controlled laboratory experiments to determine (i) whether gynes and workers of Bombus terrestris exhibit differential susceptibility to the parasite C. bombi, and (ii) whether caste-specific immune function is related to this differential susceptibility.
Materials and methods
Bumble bee caste susceptibility to C. bombi
We examined susceptibility to C. bombi in gynes and workers in two independent sets of experiments conducted in 2004 and in 2005. All Bombus terrestris colonies were purchased from Koppert B.V. (Netherlands) and supplied by Hortico Industries (Dublin, Ireland). C. bombi was isolated from gynes and workers taken from wild Irish populations of B. terrestris and kept alive in commercial hosts before experimental inoculations (Yourth and Schmid-Hempel 2006).
In both experiments, animals were removed from their colony on eclosion and transferred to individual plastic boxes (10.5 × 13 × 6 cm) with fresh pollen and 50% (vol/vol) sugar water (Apiinvert®) ad libitum. Animal collection occurred at the point in the colony life-cycle when both workers and gynes were eclosing. Animals were inoculated at 8 days post eclosion. Before inoculation faeces were checked under the microscope to confirm their parasite-free status. The parasite was isolated from the faeces of infected commercial hosts (see above) and diluted with sugar water to produce inocula of 1000 cells/μl in 2004 and 500 cells/μl in 2005 before experimental oral inoculations. In 2005, the inocula concentration was halved due to the constraint imposed by the high number of animals to be inoculated. Both concentrations, however, are known to produce successful infection in gynes and workers (Ruiz-González and Brown 2006; Brown et al. 2003b). We followed the Brown et al. (2003b) infection protocol: animals were starved for 3–4 h prior to being fed with the inoculum (inoculated animals) or with sugar water (control animals).
In 2004, we examined susceptibility in gynes and workers using eight workers and eight gynes from each of two colonies (32 animals), and all bees were inoculated. We checked the faeces of all post-inoculated animals on a daily basis for 15 days; the average first day of parasite shedding in gynes was 5 days and in workers was 4.5 days. We also quantified the infections in gynes and workers on day 8 post-inoculation. In 2005, we examined susceptibility in approximately 15 workers (15, 14 and 14 respectively) and approximately 10 gynes (10, 11 and 10 respectively) from each of three colonies (43 workers, 31 gynes). Both gynes and workers were sampled on day 8 after inoculation to check for parasite status using faeces samples under 400X magnification. We chose day 8 post-inoculation for the first check because the first important peak in the infection dynamics of C. bombi in workers happens around this day (Logan et al. 2005; Schmid-Hempel and Schmid-Hempel 1993). Data could not be recorded in a data blind way, because we were recording parasites from two completely distinct physical castes of bumblebee. In 2005, we further used molecular analyses as a back-up for infection assessment; after DNA extraction with a solution of 10% Chelex, we used the microsatellite primer CRI4 to confirm the absence of parasites in the guts (Schmid-Hempel and Reber Funk 2004); all scoring concurred between the microscopical and molecular methods, thus, those infections confirmed microscopically and molecularly acted as a positive control.
Bumble bee caste immune defence
We conducted immune analyses on the haemolymph samples taken from approximately 15 uninoculated workers (14, 14 and 15 respectively) and approximately 10 uninoculated gynes (10, 11 and 9 respectively) from each of the three colonies (43 workers, 30 gynes) in 2005 following Brown et al. (2003b). Animals were removed from their colony on eclosion and maintained as described above. At day 16 post-eclosion, we collected a sample of 10 μl of haemolymph from each insect. For each individual, the haemolymph concentrations of active phenoloxidase (PO) and the proenzyme proPO were measured. PO was quantified without further activation, while the proenzyme proPO was assayed after its activation with chymotrypsin to active PO. The same reaction mixtures were set up for gynes and workers. For PO measurements, reaction mixtures contained 20 μl of haemolymph solution (dilution 1/20; haemolymph/Sodium cacodylate/CaCl2 buffer), 140 μl of distilled water, 20 μl of phosphate buffer saline (PBS: 8.74 g NaCl; 1.78 g Na2HPO4, 2H2O; 1000 ml distilled water; pH. 6.5) and 20 μl of L-Dopa solution (4 mg/ml of distilled water). For measuring proPO, the 20 μl of haemolymph solution and the 140μl of distilled water in each microtiter plate well contained chymotrypsin (0.07 mg/ml) and were incubated for 5 min at room temperature before reading enzymatic activity. The reaction was allowed to proceed at 30 °C in a microplate reader (Versamax, Molecular Devices) for 40 min. Readings were taken every 10 s at 490 nm and analysed using SOFTmax®PRO 4.0 software (Molecular Devices). Enzyme activity was measured as the slope (Vmax value) of the reaction curve during the linear phase of the reaction (Barnes and Siva-Jothy 2000). The PO level is the quantity of enzyme invested in the response to a given challenge, while the pro-PO level is the total quantity of enzyme available.
The baseline humoural cell-free bacterial activity was measured as the antibacterial activity (zone of inhibition, ZI, on live Arthrobacter globiformis bacteria suspension (105 cells/ml) in sterile broth medium (10 g of bactotryptone, 5 g of yeast extract, 10 g of NaCl, 1000 ml of distilled water) with 1% of bacto-agar). Ten test holes (diameter: 2 mm) were made per plate, and 2 μl of the haemolymph solution (dilution 1/5; haemolymph/sodium cacodylate/CaCl2 buffer) was added per hole (Moret and Schmid-Hempel 2000). Plates were incubated overnight at 28 °C. After 24 h of bacterial incubation, the diameter of each zone of inhibition was measured. Because of potential inter-plate variation (e.g. due to variation in bacterial growth, agar thickness, etc.), the zone of inhibition for each sample was corrected across plates by always injecting the 10th hole of each plate with the same sample of Tenebrio mollitor haemolymph; therefore, this sample acted as a control.
Statistical analysis
We used a binary logistic regression to test whether caste, colony, year (equivalent to dose, as these two variables covaried) or the interactions between caste and colony, and caste and year, predicted the outcome of inoculation.
We analysed the intensity of infection on day 8 post-inoculation for workers and queens from two colonies inoculated in 2004 using a GLM with caste and colony as fixed factors.
Because Box’s test of equality of covariance matrices was failed, we could not analyse the immune data using a MANOVA-type analysis. Consequently, we used a GLM with caste and colony as fixed factors for each individual immune parameter and corrected for multiple testing using the step-up sequential Bonferroni correction (Hochberg 1988). All statistical analyses were conducted with IBM © SPSS © Statistics 24.
Results
Bumble bee caste susceptibility to C. bombi
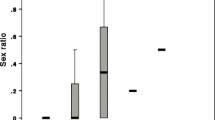
Based on binary infection prevalence outcomes, gynes were significantly less susceptible to the parasite than were workers (Wald = 28.932, P < 0.001, Exp (B) = 0.079; Fig. 1). Overall, 25.5% of gynes and 81.4% of workers became infected with C. bombi after inoculation. While in 2004, 18.8% of gynes versus 93.8% of workers became infected, and in 2005 infection ran at 29.0% of gynes and 76.7% of workers, there was no effect of year (it did not appear in the final model). Similarly, neither colony nor the interactions between caste and colony or caste by year predicted infection success. The final model, which only included caste as a predictor variable, successfully predicted 78.3% of the outcomes, a significant improvement on the 56.6% successful predictions of the null model. In contrast to these caste effects on infection success, for infected animals, there was no difference in the intensity of infection at day 8 post-inoculation in queens and workers, although there was a trend for gynes to have lower intensity infections (Caste: F1,15 = 1.005, P = 0.332; Colony: F1,15 = 0.064, P = 0.803; Fig. 2).
C. bombi intensity of infection in bumble bee castes. Average levels of infection at 8 days post-inoculation (♦) in gynes and workers from colonies A and B inoculated in 2004. The lower and upper sides of the box represent the first and third quartiles (Q1 and Q3); the line in the middle represents the median (or Q2). The dots are the mean values. The whiskers represent the minimum and maximum values
Bumble bee caste immune defence
The analysis of uninoculated bumble bees detected significant overall differences in immune function between gynes and workers for PO (F1,62 = 7.856, P = 0.007; higher levels in gynes than in workers; Fig. 3), PPO (F1,62 = 8.095, P = 0.006; higher levels in gynes than in workers; Fig. 3) and ZI (F1,62 = 8.051, P = 0.006; lower levels in gynes than in workers; Fig. 3.). Colonies varied significantly in their PO (F2,62 = 6.555, P = 0.003, with higher PO activity in colony 1; Fig. 3). After correction for multiple testing, there were no further significant effects in any immune measure of colony (PPO: F2,62 = 1.178, uncorrected P = 0.315; ZI: F2,62 = 2.529, uncorrected P = 0.088), or the colony by caste interaction (PO: F2,62 = 4.632, uncorrected P = 0.013; PPO: F2,62 = 3.357, uncorrected P = 0.041; ZI: F2,62 = 0.968, uncorrected P = 0.385).
Immune defence in bumble bee castes. a PO activity; b PPO activity; c antimicrobial activity. The lower and upper sides of the box represent the first and third quartiles (Q1 and Q3); the line in the middle represents the median (or Q2). The dots are the mean values. The whiskers represent the minimum and maximum values
Discussion
Bumble bee gynes are less susceptible to a prevalent parasite than are workers, as would be expected given the differential impact on colony fitness of C. bombi infections across the two castes. Gynes also exhibited higher levels of constitutive immunity and lower levels of baseline humoural cell-free anti-bacterial activity than workers did. While initial studies of immunity in response to parasitism by C. bombi identified constitutive immunity as important (Brown et al. 2003b), more recent studies have instead identified the role of anti-microbial peptides in defence against this parasite (e.g. Marxer et al. 2016). Thus, while higher constitutive immunity in the haemolymph may be related to parasite resistance, this remains to be shown.
In eusocial systems, protecting the colony reproductive(s) from parasites is key to colony success. While recent studies have suggested that such defence may come about through behavioural mechanisms, here, we have demonstrated that gynes are intrinsically less susceptible to parasites than their sister workers. This provides causal support for previous observations in this system (Shykoff and Schmid-Hempel 1991a; Ulrich et al. 2011; Fowler et al. 2020). In contrast, a previous study in honey bees found that queens and workers were equally susceptible to the microsporidian parasite Nosema apis (Webster et al. 2004). Unfortunately, there is no consensus on the impact of N. apis on honey bee queens (L’Arrivée 1965; Czekonska 2000; Strange and Calderone 2009), and so it is difficult to determine whether this lack of differential susceptibility contrasts with, or supports our findings.
Given the clear relationship between C. bombi and bumble bee immune function (Brown et al. 2003b; Otterstatter and Thomson 2006; Barribeau et al. 2014; Deshwal and Mallon 2014), one mechanism that could produce low gyne susceptibility may be caste-specific immune profiles. In fact, strong differences have been demonstrated in the expression of immune genes between bumble bee queens and males (Barribeau et al. 2015), and in honey bees, with a similar immune gene repertoire to that of the bumble bee (Sadd et al. 2015). Moreover, Li et al. (2018) found higher expression levels of methyltransferases genes in the fat body of the queens than in workers, which might be related to the imprinting and differential expression of immune genes in both castes. Surprisingly, given that anti-microbial peptides have been most strongly implicated in the success, or not, of C. bombi infections in bumblebees (Barribeau et al. 2014; Deshwal and Mallon 2014), gynes did not exhibit higher anti-microbial activity than their more susceptible sister workers. However, one reason for this may be that we measured immunity in the haemolymph, in uninfected individuals, rather than in the gut.
An alternative explanation for differential caste susceptibility to infection may be body size. B. terrestris exhibits strong queen-worker dimorphism, and smaller workers may require a lower parasite dose to become infected. As there is little if any overlap in body size between queens and workers, any analysis of the impact of body size is completely confounded by caste. However, using data from a previous study on workers (this study had a considerably larger sample size of workers than the current study, and thus generates a more powerful analysis; Brown et al. 2000), we found that despite a more than 2-fold range in body mass, size had no effect on whether inoculated workers became infected (binary logistic regression, bee fresh weight, B = 0.006 ± 0.059, Wald = 0.012, P = 0.913). Thus, we consider it unlikely that body size, per se, can explain our results. Within each of the annual rounds of our experiment, we used a single parasite dose, and future studies should use a range of doses to determine the thresholds for infection in queens, in comparison to workers (Ruiz-González and Brown 2006).
Lower susceptibility of gynes to C. bombi may also help to explain the seasonal dynamics of this parasite. These dynamics consist of annual epidemics, with infection of colonies occurring at low levels in spring queens and rapidly rising to nearly 100% prevalence (Imhoof and Schmid-Hempel 1999). Higher resistance to infection in summer gynes would explain the paradox of the annual drop to lower levels of infection in the spring, given that C. bombi has been found to have no or little effect on survival across hibernation (Brown et al. 2003a; Yourth et al. 2008; but see Fauser et al. 2017). In addition, the differential susceptibility to C. bombi in gynes and workers has implications for the epidemiology and evolution of the parasite. To survive from one season to the next, the parasite must infect summer gynes that will carry it through the hibernation period. This should cause selection for strains that have higher infectivity in gynes. Such selection may be facilitated by the relative ease with which workers are infected, leading to a pool of parasite strains within colonies in the summer when gynes are produced (Ulrich et al. 2011).
We note that our experiments were limited to gynes, and reproductive females go through multiple life-stages in social insect colonies. Gynes, newly-mated queens and queens who have founded a colony may well face different trade-offs between immunity, parasites and other physiological demands, such as egg-laying. In our system, mated bumblebee queens rapidly enter hibernation, and so are unlikely to be exposed to C. bombi after mating. However, post-hibernation they face significant exposure as they forage for resources before and while founding their colony. Interestingly, mating and hibernation drive changes in gyne immunity (Barribeau and Schmid-Hempel 2017; Colgan et al. 2019) and gut microbiome (Wang et al. 2019), both of which could influence interactions between gynes and C. bombi. Further work should examine their susceptibility to infection, in comparison to gynes, and its relation to immunity.
Overall, our results suggest that parasite pressure and life-history may shape different resistance phenotypes across castes within eusocial groups. Given the prevalence of parasites and the increased risk of parasitism in group-living animals, we predict that this, along with other mechanisms of “immune privilege” (Cremer et al. 2007; Cremer 2019), is likely to be a general feature of colonial organisms, e.g. eusocial insects, shrimps and naked mole rats that produce distinct reproductive and somatic progeny.
References
Alford DV (1975) Bumblebees. Davis-Poynter Ltd, London
Anderson RM, May RM (1981) The population dynamics of microparasites and their invertebrate hosts. Phil Trans R Soc Lond B 291:451–524. https://doi.org/10.1098/rstb.1981.0005
Barnes AI, Siva-Jothy MT (2000) Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): cuticular melanization is an indicator of investment in immunity. Proc Biol Sci 267:177–182. https://doi.org/10.1098/rspb.2000.0984
Barribeau SM, Schmid-Hempel P (2017) Sexual healing: mating induces a protective immune response in bumblebees. J Evol Biol 30:202–209
Barribeau SM, Sadd BM, du Plessis L, Schmid-Hempel P (2014) Gene expression differences underlying genotype-by-genotype specificity in a host-parasite system. Proc Natl Acad Sci USA 111:3496–3501. https://doi.org/10.1073/pnas.1318628111
Barribeau SM, Sadd BM, du Plessis L, Brown MJF, Buechel SD et al (2015) A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol 16:83. https://doi.org/10.1186/s13059-015-0628-y
Brown MJF, Loosli R, Schmid-Hempel P (2000) Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91:421–427. https://doi.org/10.1034/j.1600-0706.2000.910302.x
Brown MJF, Schmid-Hempel R, Schmid-Hempel P (2003a) Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. J Anim Ecol 72:994–1002. https://doi.org/10.1046/j.1365-2656.2003.00770.x
Brown MJF, Moret Y, Schmid-Hempel P (2003b) Activation of host constitutive immune defence by an intestinal trypanosome parasite of bumble bees. Parasitology 126:253–260. https://doi.org/10.1017/S0031182002002755
Colgan TJ, Finlay S, Brown MJF, Carolan JC (2019) Mating precedes selective immune priming which is maintained throughout bumblebee queen diapause. BMC Genomics 20:959
Cordes N, Huang W-F, Strange JP, Cameron SA, Griswold TL, Lozier JD, Solter LF (2012) Interspecific geographic distribution and variation of the pathogens Nosema bombi and Crithidia species in United States bumble bee populations. J Inv Pathol 109:209–216. https://doi.org/10.1016/j.jip.2011.11.005
Côté IM, Poulin R (1995) Parasitism and group size in social mammals: a meta-analysis. Behav Ecol 6:159–195. https://doi.org/10.1093/beheco/6.2.159
Cremer S (2019) Social immunity in insects. Curr Biol 29:R425–R473. https://doi.org/10.1016/j.cub.2019.03.035
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:R693–R702. https://doi.org/10.1016/j.cub.2007.06.008
Czekonska K (2000) The influence of Nosema apis on young honeybee queens and transmission of the disease from queens to workers. Apidologie 31:701–706
Deshwal S, Mallon EB (2014) Antimicrobial peptides play a functional role in bumblebee anti-trypanosome defense. Dev Comp Immunol 42:240–243. https://doi.org/10.1016/j.dci.2013.09.004
Duffy JE (1996) Eusociality in a coral-reef shrimp. Nature 381:512–514. https://doi.org/10.1038/381512a0
Fauser A, Sandrock C, Neumann P, Sadd BM (2017) Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol Entomol 42:306–314. https://doi.org/10.1111/een.12385
Fowler AE, Stone EC, Irwin RE, Adler LS (2020) Sunflower pollen reduces a gut pathogen in worker and queen but not male bumble bees. Ecol Entomol 45:1318–1326. https://doi.org/10.1111/een.12915
Giacomini JJ, Leslie J, Tarpy DR, Palmer-Young EC, Irwin RE, Adler LS (2018) Medicinal value of sunflower pollen against bee pathogens. Sci Rep 8:14394. https://doi.org/10.1038/s41598-018-32681-y
Hamilton WD (1987) Kinship, recognition, disease, and intelligence: constraints of social evolution. In: Itô Y, Brown JL, Kikkawa J (eds) Animal Societies: Theories and Facts. Japan Scientific Societies Press, Tokyo, pp 81–102
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802
Hughes WOH, Bot ANM, Boomsma JJ (2009) Caste-specific expression of genetic variation in the size of antibiotic-producing glands of leaf-cutting ants. Proc R Soc B 277:609–615. https://doi.org/10.1098/rspb.2009.1415
Imhoof B, Schmid-Hempel P (1999) Colony success of the bumble bee, Bombus terrestris, in relation to infections by two protozoan parasites, Crithidia bombi and Nosema bombi. Insect soc 46:233–238. https://doi.org/10.1007/s000400050139
Jarvis JUM (1981) Eusociality in a mammal – cooperative breeding in naked mole-rat colonies. Science 212:571. https://doi.org/10.1126/science.7209555
Keller L, Genoud M (1997) Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature 389:958–960. https://doi.org/10.1038/40130
Koch SI, Groh K, Vogel H, Hannson BS, Kleineidam CJ et al (2013) Caste-apecific expression patterns of immune response and chemosensory related genes in the leaf-cutting ant, Atta vollenweideri. PLoS ONE 8(11):e81518. https://doi.org/10.1371/journal.pone.0081518
Krause J, Ruxton GD (2002) Living in groups. Oxford Series in Ecology and Evolution, Oxford University Press, Oxford
L’Arrivée JCM (1965) Tolerance of honey bees to nosema disease. J Invert Pathol 7:408–413. https://doi.org/10.1016/0022-2011(65)90114-X
Li B, Hou L, Zhu D, Xu X, An S, Wang X (2018) Identification and caste-dependent expression patterns of DNA methylation associated genes in Bombus terrestris. Sci Rep 8:2332. https://doi.org/10.1038/s41598-018-20831-1
LoCascio GM, Aguirre L, Irwin RE, Adler LS (2019) Pollen from multiple sunflower cultivars and species reduces a common bumblebee gut pathogen. R Soc Open Sci 6:190279. https://doi.org/10.1098/rsos.190279
Logan A, Ruiz-González MX, Brown MJF (2005) The impact of host starvation on parasite development and population dynamics in an intestinal trypanosome parasite of bumble bees. Parasitology 130:637–642. https://doi.org/10.1017/S0031182005007304
Marxer M, Vollenweider V, Schmid-Hempel P (2016) Insect antimicrobial peptides act synergistically to inhibit a trypanosome parasite. Phil Trans R Soc B 371:20150302. https://doi.org/10.1098/rstb.2015.0302
Maynard Smith J, Szathmáry E (1995) The major transitions in evolution. W. H, Freeman, New York
Moret Y, Schmid-Hempel P (2000) Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168. https://doi.org/10.1126/science.290.5494.1166
Nigam Y, Maudlin I, Welburn S, Ratcliffe NA (1997) Detection of phenoloxidase activity in the hemolymph of tsetse flies, refractory and susceptible to infection with Trypanosoma brucei rhodesiense. J Invertebr Pathol 69:279–281. https://doi.org/10.1006/jipa.1996.4652)
Otterstatter MC, Thomson JD (2006) Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology 133:749–761. https://doi.org/10.1017/S003118200600120X
Otterstatter MC, Gegear RJ, Colla SR, Thomson JD (2005) Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav Ecol Sociobiol 58:383–389. https://doi.org/10.1007/s00265-005-0945-3
Rosengaus RB, Traniello JFA (1993) Disease risk as a cost of outbreeding in the termite Zootermopsis angusticollis. Proc Natl Acad Sci USA 90:6641–6645. https://doi.org/10.1073/pnas.90.14.6641
Ruiz-González MX, Brown MJF (2006) Males vs workers: testing the assumptions of the haploid susceptibility hypothesis in bumble bees. Behav Ecol Sociobiol 60:501–509. https://doi.org/10.1007/s00265-006-0192-2
Sadd BM, Barribeau SM, Bloch G et al (2015) The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol 16:76. https://doi.org/10.1186/s13059-015-0623-3
Schmid MR, Brockmann A, Pirk CWW, Stanley DW, Tautz J (2008) Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J Insect Physiol 54:439–444. https://doi.org/10.1016/j.jinsphys.2007.11.002
Schmid-Hempel P (2001) On the evolutionary ecology of host-parasite interactions: addressing the question with regard to bumblebees and their parasites. Naturwissenschaften 81:147–168. https://doi.org/10.1007/s001140100222
Schmid-Hempel P, Heeb D (1991) Worker mortality and colony development in bumblebees, Bombus lucorum (L.) (Hymenoptera, Apidae). Mitteil Schw Entomol Gesell 64:93–108. https://doi.org/10.5169/seals-402434
Schmid-Hempel P, Reber Funk C (2004) The distribution of genotypes of the trypanosome parasite, Crithidia bombi, in populations of its host, Bombus terrestris. Parasitology 129:147–158. https://doi.org/10.1017/S0031182004005542
Schmid-Hempel R, Schmid-Hempel P (1993) Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav Ecol Sociobiol 33:319–327. https://doi.org/10.1007/BF00172930
Schmid-Hempel R, Schmid-Hempel P (2000) Female mating frequencies in Bombus spp. from Central Europe. Insectes soc 47:36–41. https://doi.org/10.1007/s000400050006
Shykoff JA, Schmid-Hempel P (1991a) Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie 22:117–125. https://doi.org/10.1051/apido:19910204
Shykoff JA, Schmid-Hempel P (1991b) Parasites delay worker reproduction in bumblebees – consequences for eusociality. Behav Ecol 2:242–248. https://doi.org/10.1093/beheco/2.3.242
Strange JP, Calderone NW (2009) Evaluation of apicultural characteristics of first-year colonies initiated from packaged honey bees (Hymenoptera: Apidae). J Econ Entomol 102:485–492. https://doi.org/10.1603/029.102.0204
The Honeybee Genome Sequencing Consortium (2006) Insights into social insects from the genome of the honeybee, Apis mellifera. Nature 443:931–949. https://doi.org/10.1038/nature05260
Ulrich Y, Sadd BM, Schmid-Hempel P (2011) Strain filtering and transmission of a mixed infection in a social insect. J Evol Biol 24:354–362
Wang L, Wu J, Li K, Sadd BM, Guo Y et al (2019) Dynamic changes of gut microbial communities of bumble bee queens through important life stages. Msystems 4:e00631–e00619
Webster TC, Pomper KW, Hunt G, Thacker EM, Jones SC (2004) Nosema apis infection in worker and queen Apis mellifera. Apidologie 35:49–54. https://doi.org/10.1051/apido:2003063
Wilson EO (1971) The Insect Societies. Harvard University Press, Cambridge, Massachusetts
Yourth CP, Schmid-Hempel P (2006) Serial passage of the parasite Crithidia bombi within a colony of its host, Bombus terrestris, reduces success in unrelated hosts. Proc R Soc B 273:655–659. https://doi.org/10.1098/rspb.2005.3371
Yourth CP, Brown MJF, Schmid-Hempel P (2008) Effects of natal and novel Crithidia bombi (Trypanosomatidae) infections on Bombus terrestris hosts. Insectes soc 55:86–90. https://doi.org/10.1007/s00040-007-0974-1
Acknowledgements
We thank R. M. Falcao and S. Cornet for the help in the laboratory and technical support and two anonymous referees and the editor for their comments on our manuscript. This work complied with the laws governing animal research in Ireland.
Availability of data and material
Data are deposited at Dryad as follows: Ruiz-González, Mario X.; Kelly, Michael; Moret, Yannick; Brown, Mark J. F. (2021), parasite resistance and immunity across female castes in a social insect, Dryad, Dataset, https://doi.org/10.5061/dryad.sj3tx964.
Code availability
Not applicable.
Funding
This study was supported by an Enterprise Ireland grant to M. J. F. B., a Ulysses grant to M. J. F. B. and Y. M., and Y. M. was supported by the Centre National de la Recherche Scientifique (C.N.R.S.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
MXRG and MJFB designed the study. YM provided training in immune methods. MXRG and MK conducted the experimental work. MXRG conducted the statistical analyses and wrote the first draft of the manuscript. All authors contributed to the final draft.
Corresponding authors
Ethics declarations
Ethics approval
This work complied with the laws governing animal research in Ireland.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
This article is a contribution to the Topical Collection Sociality and Disease – Guest Editors: Rebeca Rosengaus, James Traniello, and Theo Bakker
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruiz-González, M.X., Kelly, M., Moret, Y. et al. Parasite resistance and immunity across female castes in a social insect. Behav Ecol Sociobiol 76, 56 (2022). https://doi.org/10.1007/s00265-022-03162-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03162-0