Abstract
Eusocial insects can employ various behavioural and physiological disease defences to avoid, resist and tolerate pathogen infections in their closely related and packed colonies, termed social immunity. Recent studies have shown that several molecules serve insect social immunity, including chemical odours, insect venoms, immune-related proteins, etc. However, whether and how microRNAs (miRNAs), whose precursors are processed by Dicer-1, drive social immunity in insect colonies is still unknown. Here, we used a ‘host–pathogen’ system (host: Reticulitermes chinensis; pathogen: Metarhizium anisopliae) to explore the impact of miRNAs on social immunity in termite colonies. We found that RNAi-mediated silencing of Dicer-1 led to decreased miRNA concentration, significantly inhibited carbohydrate and energy metabolism and affected other life processes, such as the immune response and oxidation–reduction reactions, in whole body of the termite. In behavioural defence, silencing Dicer-1 significantly diminished defensive social behaviours such as locomotion, grooming, cannibalism and burial in termite groups when encountering fungal contamination. In physiological defence, Dicer-1 silencing and miR-71-5 stimulation resulted in significantly decreased antifungal activities of termites. Furthermore, both Dicer-1-silenced and miR-71-5 stimulant-treated termite groups exhibited a high level of mortality during fungal contamination. Our findings demonstrated the important role of miRNAs in shaping social immunity in termite colonies, providing insights necessary to understand the potential mechanisms underlying behavioural and physiological disease defences in insects and hence laying the groundwork for miRNA-based pest control.
Similar content being viewed by others
Key message
-
miRNAs mediated gene expressions related to social immune systems.
-
miRNAs affected the behavioural disease defences in termite groups.
-
miRNAs influenced the physiological disease defence in termite individuals.
-
miRNA-based termiticides could be considered a novel accesses to biological pest control.
Introduction
Termites are economically important pests, costing 30 billion dollars worldwide, imposing global damage to crops, trees, wooden structures, and cellulose materials. Chemical pesticides provide an effective control avenue for termite pests, but they also create serious problems, threatening human health and the environment. Biological control is a valuable alternative that can address these problems. Several entomopathogens, such as Metarhizium and Beauveria, have been successfully applied for biological pest control, which is reasonable and effective (Verma et al. 2009; Kumar and Upadhyay 2021). However, the biological control of termites is unsatisfactory, mainly due to the unique disease defences in termite colonies.
Termites, being eusocial insects, have evolved collective behavioural and individual physiological defences to counteract the high risk of the spread of infectious pathogens among closely packed and closely related nestmates (termed ‘social immunity’) (Van Meyel et al. 2018; Liu et al. 2019a). It is well documented that termites deploy a wide repertoire of behavioural defences: avoidance is the first line of defence and helps to prevent termites from entering polluted areas (Yanagawa et al. 2015); alarm behaviour is exhibited with rapid longitudinal oscillatory movement to warn nestmates about the presence of pathogens (Rosengaus et al. 1999); grooming towards pathogen-contaminated nestmates can efficiently remove pathogens from nestmate cuticles (Liu et al. 2019b); burial and cannibalistic behaviours limit pathogen transmission from corpses to susceptible nestmates (Sun et al. 2016). Physiological responses such as immune responses and oxidation–reduction reactions also serve termite disease defence. Immune responses include humoral immunity-mediated antimicrobial peptides (AMPs) and cellular immunity-mediated phagocytosis and encapsulation, which are able to inhibit the replication and dissemination of pathogens in the insect body cavity (Hussain et al. 2013; Liu et al. 2015; Lopez-Uribe et al. 2016; Hong et al. 2018). Oxidation–reduction reactions mitigate the damage caused by toxins and reactive oxygen species (ROS) during pathogen infections, contributing to insect tolerance (Liu et al. 2015; Zhao et al. 2020; Zhou et al. 2021). In addition, previous studies on termite disease defences have shown that some gut symbionts were an important component of the host physiological defence (Rosengaus et al. 2014). Some AMPs could be employed as external disinfectants on the cuticle surface and in nest materials when in conjunction with behaviours such as grooming and nesting (Bulmer et al. 2009; Hamilton and Bulmer 2012). Therefore, weakening termite behavioural and physiological defences may be the key step to enhance the biocontrol effect of termites.
The molecular basis of social immunity has been reported in a growing number of studies. Several molecules, including chemical odours, external secretions, antibiotics, immune proteins, and chemoreceptors serving insect behavioural and physiological defences, have been identified by multiple omics techniques and gas/liquid chromatography–mass spectrometry (Seipke et al. 2011; Hussain et al. 2013; Terrapon et al. 2014; Liu et al. 2015; Sun et al. 2016; He et al. 2018). In termite groups, musty odour from pathogens induces enhanced grooming behaviour (Yanagawa et al. 2011). Death cues from termite corpses induce either cannibalism or burial behaviours (Sun et al. 2016). External secretions from the frontal and salivary glands and beneficial microorganisms-mediated antimicrobial activity help termites protect themselves, nestmates and even colonies (Bulmer et al. 2009; Rosengaus et al. 2000, 2014; Chouvenc et al. 2013). For the genetic and biochemical mechanisms driving insect social immunity, RNAi and gene editing technologies could be used. In termites, RNAi-mediated silencing of the termicin, gram-negative binding protein 2 (GNBP2), selenium-binding protein, and transglutaminase (TG) genes has been demonstrated to significantly decrease the antifungal activity of termites and hence increase infection mortality (Hamilton and Bulmer 2012; Zhao et al. 2020; Zhou et al. 2021). Isocitrate dehydrogenase (IDH) could influence termite metabolism, and its dysregulation induces increased apoptotic lesions, leading to high levels of infections and mortalities (Liu et al. 2020). In addition, GNBP2 and TG also serve the behavioural defence of termites, influencing cannibalistic and grooming behaviours, respectively (Zhao et al. 2020; Esparza-Mora et al. 2020). However, the molecular basis of social immunity has yet to be clearly understood, especially the molecular mechanism driving the complicated social behaviours in response to infectious pathogens. Furthermore, the genetic and biochemical mechanism of social immunity focuses on the coding RNA driving insect social immunity, but whether noncoding RNAs such as miRNAs take part in the regulation of insect social immunity is still unclear.
Dicer-1, an RNase III endonuclease, is essential for the final step of miRNA biosynthesis. miRNAs are endogenous, 18–25 nt, noncoding RNAs that negatively regulate gene expression at the posttranscriptional level by base pairing between the seed region in miRNAs and the matched region in target mRNAs (Gomez-Orte and Belles 2009; Wang et al. 2013; Yang et al. 2014; Lucas et al. 2015). As miRNA production depends on Dicer-1, the functions of miRNAs in modulating the biological processes of insects can be studied by functional losses of Dicer-1. By using Dicer-1 mutants of fruit flies, miRNAs have been proven to function in embryogenesis and olfactory neuron morphogenesis (Lee et al. 2004; Berdnik et al. 2008). By RNAi-mediated silencing of Dicer-1, researchers have verified that miRNAs play a key role in regulating developmental processes in insect metamorphosis, such as those in cockroaches and locusts (Gomez-Orte et al. 2009; Wang et al. 2013). Moreover, miRNAs are also involved in modulating insect behaviours. miR-8 and miR-429 regulate virus-induced climbing behaviour in cotton bollworms by directly controlling BrZ2 expression (Zhang et al. 2018). miR-133 regulates dopamine synthesis and hence inhibits behavioural aggregation in the locust (Yang et al. 2014). Based on such evidence for the function of miRNAs in insect physiology and behaviour, we aimed to determine the effect of miRNAs on termite physiology and behaviour involved in social immunity by using a ‘host–pathogen’ system (host: Reticulitermes chinensis; pathogen: Metarhizium anisopliae). Our study mainly included three aspects of the research on social immunity: the effect of miRNAs on (1) the in vivo molecules driving social immunity; (2) the behavioural defensive response, such as locomotion in fungus-contaminated areas, grooming towards fungus-contaminated nestmates and cannibalism/burial towards fungus-contaminated corpses; and (3) the physiological defence response, such as antifungal activity. Furthermore, we tested the effect of miRNA dysregulation on the mortality of fungus-contaminated termites to evaluate the effect of miRNAs on biological control of the termites. Our study revealed the potential mechanism underlying behavioural and physiological disease defences and novel targets for pest control.
Material and methods
Termites
All experimental termites in our study were workers of the termite R. chinensis. They were collected from Shizi Hill in Wuhan City, Hubei Province, China. Termites were reared in plastic containers (40 × 20 × 20 cm) containing small pine blocks in the laboratory at a temperature of 25 ± 1 °C, 80% relative humidity, and 24 h of darkness.
Entomopathogens, contaminated termites and corpses
The entomopathogenic fungus M. anisopliae (strain IBCCM321.93) was cultivated on potato dextrose agar (PDA) for 2 weeks at 25 ± 1 °C, 80% relative humidity, and 24 h of darkness. The fungal conidia were suspended using 1% Tween 80 and stored at a temperature of 4 °C for a maximum of 3–4 weeks. Before each experiment, the conidial suspension was tested for germination. We found that the conidial suspension used for contaminating termites had a germination rate of more than 90%.
To prepare fungus-contaminated termites, the abdomens of termites were contaminated by a 0.3 μL droplet of conidial suspension (108 conidia/mL) using a pipette (0.1–2.5 μL, Transferpette). Then, termites contaminated with the fungus were immediately refrigerated at 4 °C for one hour to inhibit their movement to precipitate fungal conidia on their cuticles (Liu et al. 2015).
To prepare fungus-contaminated corpses, termites were collected in a 1.5 mL centrifuge tube and then frozen in liquid nitrogen for 20 s to kill the termites. The corpses were immersed in the conidial suspension (108 conidia/mL) and then placed in a sterile petri dish with moist filter paper for 0 and 2 d of cultivation (Sun et al. 2016).
Synthesis of dsRNA and the miRNA simulant
Dicer-1 fragment (1697 bp; Supplementary material 1-Text S1) was amplified by specific primers (forward: 5′-CTG CGA CAG ATC ATT GCA CG-3′; reverse: 5′-CAC TGG CTG TTT TGG CAC TC-3′), and their PCR products were purified using the AxyPrepTM DNA Gel Extraction Kit (Axygen Scientific, USA), cloned into the pMD 18-T vector (TaKaRa, Japan) and then transformed using a DH5α chemically competent cell (Tsingke Biotechnology, China). A single colony from lysogeny broth (LB) medium with ampicillin was sent to Tsingke Biotechnology Co. Ltd. for sequencing. The sequence alignment showed that our fragment was Dicer-1. The T7 promoter sequence (5′-GGA TCC TAA TAC GAC TCA CTA TAG G-3′) was added to the 5′ end of the amplification primers of Dicer-1 (519 bp; Forward: 5′-GTG ATG CTG GAG TTG GGT TT-3′; Reverse: 5′-AGA ATG AGT CGC CCA ATG TC-3′) and GFP (467 bp; Forward: 5′-CTT GAA GTT GAC CTT GAT GCC-3′; Reverse: 5′-TGG TCC CAA TTC TCG TGG AAC-3′) dsRNA templates (Supplementary material 1-Text S1). The PCR products of the dsRNA templates were extracted with hydroxybenzene/chloroform/isoamyl alcohol (25:24:1) (Solarbio, China). dsRNA was generated using a T7 RNAi Transcription Kit (Vazyme Biotech, China) and then purified by hydroxybenzene/chloroform/isoamyl alcohol (50:49:1) (Solarbio). Finally, dsRNA was assessed using agarose gel electrophoresis and a NanoDrop 2000 (Thermo Scientific, USA) and stored at − 80 °C.
miR-71-5 simulant was a small double-strand RNA designed according to the miR-71-5 sequence (21 nt, Supplementary material 1-Text S1). The template of the miR-71-5 simulant consisted of two double-stranded DNAs containing the T7 promoter: one was generated by oligonucleotide A1 (5′-GAT CAC TAA TAC GAC TCA CTA TAG GGT GAA AGA CAT GGG TAA TGA GAA A-3′) and oligonucleotide A2 (5′-TTT CTC ATT ACC CAT GTC TTT CAC CCT ATA GTG AGT CGT ATT AGT GAT C-3′) using touchdown PCR; the other was generated by oligonucleotide B1 (5′-GAT CAC TAA TAC GAC TCA CTA TAG GGT CTC ATT ACC CAT GTC TTT CAA A-3′) and oligonucleotide B2 (5′-TTT GAA AGA CAT GGG TAA TGA GAC CCT ATA GTG AGT CGT ATT AGT GAT C-3′) using touchdown PCR. A control simulant was designed according to the GFP sequence (19 nt, Supplementary material 1-Text S1). The template of the control simulant was also acquired by two double-stranded DNAs containing the T7 promoter: one was generated by oligonucleotide C1 (5′-GAT CAC TAA TAC GAC TCA CTA TAG GGG CAA GCT GAC CCT GAA GTT AA-3′) and oligonucleotide C2 (5′-TTA ACT TCA GGG TCA GCT TGC CCC TAT AGT GAG TCG TAT TAG TGA TC-3′) using touchdown PCR; the other was generated by oligonucleotide D1 (5′-GAT CAC TAA TAC GAC TCA CTA TAG GGA ACT TCA GGG TCA GCT TGC AA-3′) and oligonucleotide D2 (5′-TTG CAA GCT GAC CCT GAA GTT CCC TAT AGT GAG TCG TAT TAG TGA TC-3′) using touchdown PCR. The miR-71-5 and control simulants were generated using a T7 RNAi Transcription Kit and then extracted by hydroxybenzene/chloroform/isoamyl alcohol (50:49:1). The extract was assessed using agarose gel electrophoresis and a NanoDrop 2000 and stored at − 80 °C.
dsRNA feeding
Termites were prestarved for 12 h before being fed (Zhou et al. 2008; Hamilton and Bulmer 2012). A total of 18 termites were placed in a cell petri dish (D = 35 mm) with a piece of filter paper (D = 18 mm) that was moistened by 60 μL of RNase-free water containing 80 μg dsDicer-1, 80 μg dsGFP, 60 μg miRNA simulant, or 60 μg control simulant. For Dicer-1 dsRNA feeding bioassays, termites were allowed to ingest the moistened paper for 24 h before the experiments. GFP-treated termites were regarded as controls. For miRNA simulant feeding bioassays, termites were allowed to ingest the moistened paper for 48 h before the experiments. Control simulant-treated termites were regarded as controls.
RT-qPCR
Five or six replicates from three colonies were used for gene expression using RT-qPCR. Three termites per replicate per treatment were pooled for total RNA extraction using the Direct-zol™ RNA MiniPrep Kit (Zymo Research, USA). The purity and concentration of the extracted RNA were determined using a NanoDrop 2000. RNA was converted to cDNA using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TakaRa, Japan). RT-qPCR was performed with a QuantStudio™ 3 Real-Time PCR System (Thermo Scientific) using ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech). The specific primers are listed in Supplementary material 2-Table S1.
miRNA concentration
Six replicates from three colonies were used to determine the concentration of miRNAs using a MiPure Cell/Tissue miRNA Kit (Vazyme Biotech, China). Three termites per replicate per treatment were pooled for miRNAs. The purity and concentration of the extracted miRNAs were determined using a NanoDrop 2000.
De novo sequencing and transcriptome analysis flow
Fifteen termites from three colonies per treatment were pooled for total RNA extraction using TRIzol Reagent (Invitrogen Life Technologies, USA). The concentration, quality and integrity of the total RNA were determined using a NanoDrop spectrophotometer (Thermo Scientific). Sequencing libraries were generated using the TruSeq Stranded mRNA Sample Prep Kit (Illumina, USA) and sequenced on a HiSeq platform (Illumina) by Shanghai Personal Biotechnology Cp. Ltd. (China). Sequencing data were filtered by Cutadapt (v1.15) software. For the transcriptome sequencing project without a reference genome, Trinity (v2.5.1) software was used to montage clean reads for the transcripts for later analysis. NR (NCBI non-redundant protein sequences), GO (Gene Ontology), KEGG (Kyoto Encyclopedia of Genes and Genome), EggNOG (Evolutionary genealogy of genes: Non-supervised Orthologous Groups), and Swiss-Prot databases were used for gene functional annotation. Furthermore, DESeq (1.30.0) was used to analyse the differentially expressed genes under the condition of |log2FoldChange(FC)|> 1 and p < 0.05. The genes were then mapped to GO terms, and the terms with significant enrichment were calculated by hypergeometric distribution under the condition of p < 0.05 to reveal the possible functions of the candidate genes. Additionally, the genes were mapped to KEGG pathways to determine their possible functions.
Locomotion
Nine replicates from three colonies were analysed to compare the movement distance of Dicer-1-silenced vs. GFP-treated termites. After one day of oral feeding of dsDicer-1 or dsGFP, one termite per replicate per treatment was placed in a new cell petri dish (D = 90 mm) with a piece of filter paper moistened by the conidial suspension (108 conidia/mL). Then, we used a black marker to trace the movement of the termite on a transparent lid for 10 s. The movement distance was measured using grid paper (1 × 1 mm).
Grooming
Nine replicates from three colonies were analysed to compare the number of grooming behaviours between Dicer-1-silenced and GFP-treated termites. After one day of oral feeding of dsDicer-1 or dsGFP, three termites per replicate per treatment were placed in a new cell petri dish (D = 35 mm) with a piece of moistened filter paper (D = 35 mm) and then contaminated with fungal conidia (108 conidia/mL). Behaviours were video-recorded for 15 min using an HD digital camera (SONY, Japan). The videos were scanned every 10 s to observe whether grooming behaviours (termite mouths towards nestmate bodies) occurred between fungus-contaminated termites.
Cannibalism
Nine replicates from three colonies were analysed for the effect of Dicer-1 vs. GFP on the cannibalistic behaviour towards fungus-contaminated corpses. Six termites per replicate per treatment were placed in a cell petri dish (D = 35 mm) with a piece of moist filter paper (D = 18 mm) containing dsDicer-1 or dsGFP. They were allowed one day of oral feeding of dsRNA, and then, one fungus-contaminated corpse incubated for 0 d was placed into the cell petri dish where the six treated termites were reared. After one day of rearing together, the fungus-contaminated corpse was either completely eaten (high test score = 2), partially eaten (low test score = 1), or not eaten (no test score = 0) (Supplementary material 1-Fig. S1) by the six termites.
Burial
Nine replicates from two colonies were analysed for the effect of Dicer-1 vs. GFP on the burial behaviour towards fungus-contaminated corpses. Six termites per replicate per treatment were placed in a cell petri dish (D = 35 mm) with a piece of moist filter paper (D = 18 mm) containing dsDicer-1 or dsGFP. They were allowed one day of oral feeding of dsRNA. Then, one fungus-contaminated corpse incubated for 2 d and soil were added to the petri dish in which the six treated termites were reared. After one day of rearing together, the fungus-contaminated corpse was either completely buried (high test score = 2), partially buried (low test score = 1), or not buried (no test score = 0) (Supplementary material 1-Fig. S2) by the six termites.
Antifungal activity
Six replicates from three colonies were analysed for the impact of Dicer-1 and miR-71-5 on the ability of termites to inhibit fungal growth. Five termites per replicate per treatment were pooled and crushed using liquid nitrogen and then dissolved in 100 μL of 0.9% saline. The homogenate was centrifuged at 6000×g for five minutes at 4 °C to yield 80 μL of supernatant. Then, the supernatant was centrifuged again to yield 50 μL of supernatant. For antifungal activity, a 96-well microplate was used to measure the absorbance of samples, growth controls and standard blanks to calculate the reduction of the fungal growth: (1) 50 μL of potato dextrose (PD), 2 μL of the fungal conidia (108 conidia/mL), and 5 μL of the supernatant per well were mixed for the sample; (2) 50 μL of potato dextrose (PD), 2 μL of the fungal conidia (108 conidia/mL), and 5 μL of 0.9% saline per well were mixed for the fungal growth control; (3) 50 μL of potato dextrose (PD) and 7 μL of 0.9% saline per well were mixed for the standard blank. After 24 h of cultivation in a constant temperature shaker (150 rpm at 25 ± 1 °C), measurement was performed in a microplate spectrophotometer (600 nm; Thermo Scientific).
Survival
To determine the effect of oral feeding of dsDicer-1 on termite survival, termites from three colonies were deployed to determine the survival of Dicer-1-silenced (42 termites in total, 14 termites per colony) and GFP-treated (42 termites in total, 14 termites per colony) termites during fungal contamination. For controls, 56 termites from two colonies were deployed to determine the survival of Dicer-1-silenced (28 termites in total, 14 termites per colony) and GFP-treated termites (28 termites in total, 14 termites per colony). When performing the experiments, seven termites were reared in a cell petri dish (D = 35 mm) with a piece of filter paper (D = 18 mm) moistened with RNase-free water containing dsDicer-1 or dsGFP, while termites were contaminated with fungus and then reared for ten days for daily observation of their survival. To determine the effect of oral feeding of miR-71-5 simulant on termite survival, termites from three colonies were deployed to determine the survival of miR-71-5 (42 termites in total, 14 termites per colony) and control (42 termites in total, 14 termites per colony) simulant-treated termites during fungal contamination. Additionally, 56 termites from two colonies were deployed to determine the survival of miR-71-5 (28 termites in total, 14 termites per colony) and control (28 termites in total, 14 termites per colony) simulant-treated termites. When performing the experiments, seven termites were reared in a cell petri dish (D = 35 mm) with a piece of filter paper (D = 18 mm) moistened with RNase-free water containing miR-71-5 simulant or control simulant. After two days of oral feeding, termites were contaminated with fungus and then reared for ten days for daily observation of their survival. Dead termites were removed in time.
Statistical analysis
All data analyses were conducted in IBM SPSS, version 19. The Shapiro–Wilk test was used to detect whether datasets were normally distributed. Given that the gene expression, cannibalism, and burial data were abnormally distributed, the Wilcoxon test was used. Alternatively, data from the miRNA concentration, locomotion, grooming, and antifungal activity were normally distributed, and a paired t test was used. Survival was analysed using the Kaplan–Meier method for the lifespans under the different treatment conditions. The significance level was p < 0.05 in this study.
Results
mRNA expression profile of Dicer-1-silenced termites
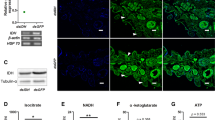
To determine the effect of Dicer-1 on the gene expression profile of termites, mRNA sequencing of Dicer-1-silenced termites was performed. After 1 day of oral feeding of dsDicer-1, Dicer-1 expression (Fig. 1A; n = 6, p < 0.05; Wilcoxon test) and hence miRNA concentration (Fig. 1B; df = 5, p < 0.05; paired t test) were significantly decreased in termites. A total of 1262 mRNAs were significantly altered in the whole bodies of Dicer-1-silenced termites (upregulated: 562 mRNAs; downregulated: 700 mRNAs; Fig. 1C and Supplementary material 2-Table S2). The differentially expressed genes (DEGs) were mapped to the salivary secretion, glycolysis, citrate cycle, and 29 other KEGG pathways (Fig. 1D–F and Supplementary material 2-Table S3). In addition, the DEGs were clustered into the ATP generation from ADP, structural constituent of cuticle, defense response, glutathione transferase activity, oxidation–reduction process, and 171 other GO terms (Fig. 2 and Supplementary material 2-Table S4).
Gene expression profile in the whole bodies of Dicer-1-silenced termites. A Dicer-1 expression. The data are shown as the mean ± SEM. *p < 0.05. B The concentration of miRNAs. The data are shown as the mean ± SEM. *p < 0.05. C The number of differentially expressed genes (DEGs). D The DEGs mapped to salivary secretion. ATP1B, Sodium/potassium-transporting ATPase subunit beta; ATP2B, Plasma membrane calcium-transporting ATPase 1; PKC, Protein kinase C alpha type; CALM, Calmodulin-like protein; LYZ, Lysozyme C. E The DEGs mapped to glycolysis. GPI, Glucose-6-phosphate isomerase; PFKC, ATP-dependent 6-phosphofructokinase; TIM, Triosephosphate isomerase; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; PGK, Phosphoglycerate kinase; MINPP1, Multiple inositol polyphosphate phosphatase 1; GPMA, 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase; ENO, Enolase. F DEGs mapped to citrate cycle. PCK, phosphoenolpyruvate carboxykinase [GTP]; PYC, pyruvate carboxylase; MDH1, malate dehydrogenase; SUCD, succinate–CoA ligase [ADP-forming]. Red letters indicate upregulation; blue letters indicate downregulation
RT–qPCR verification of DEGs from mRNA-seq
The glycolysis, citrate cycle, and ATP generation from ADP were related to carbohydrate and energy metabolism, in which eight DEGs (glyceraldehyde-3-phosphate dehydrogenase, 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase, enolase, triosephosphate isomerase, ATP-dependent 6-phosphofructokinase, phosphoenolpyruvate carboxykinase [GTP], succinate–CoA ligase [ADP-forming] subunit alpha-2, and malate dehydrogenase) were verified to be significantly downregulated after Dicer-1 silencing (Fig. 3A–H; n = 6, p < 0.05; Wilcoxon test), suggesting disorders of glycolysis, the citrate cycle, ATP generation, and hence carbohydrate and energy metabolism in the whole bodies of Dicer-1-silenced termites.
DEGs (defensin and terminicin) in the defence response encoded AMPs. Expression of these genes was significantly increased in Dicer-1-silenced termites compared to that in GFP-treated termites (Fig. 3I, J; n = 5 or 6, p < 0.05; Wilcoxon test). In the oxidation–reduction process, cytochrome P450 9e2 and peroxiredoxin-4 were significantly altered after Dicer-1 silencing in termites (Fig. 3K, L; n = 6, p < 0.05; Wilcoxon test). These results implied an important effect of Dicer-1 on the immune response and oxidation–reduction reaction in whole-body of the termites.
The effect of Dicer-1 on behavioural disease defences in termite groups
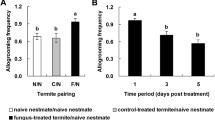
To determine the effect of Dicer-1 on the behavioural response to fungal contamination, we tested four important behavioural defences, locomotion, grooming, cannibalism, and burial, in Dicer-1-silenced termites. Our results showed that locomotion (Fig. 4A, t = 7.013, df = 8, p < 0.01; paired t test; Supplementary material 1-Fig. S3 and Video S1), grooming (Fig. 4B, t = 5.949, df = 8, p < 0.01; paired t test; Supplementary material 1-Fig. S4 and Video S2), cannibalistic (Fig. 4C , n = 9, p < 0.05; Wilcoxon test; Supplementary material 1-Fig. S1 and Video S3) and burial (Fig. 4D; n = 9, p < 0.05; Wilcoxon test; Supplementary material 1-Fig. S2 and Video S4) behaviours were significantly reduced in Dicer-1-silenced termites compared to GFP-treated termites, suggesting an important role of Dicer-1 in driving behavioural disease defences in termite colonies.
The effect of Dicer-1 and miR-71-5 on antifungal abilities in termites
To determine the effect of miRNAs on the physiological response to fungal contamination, we tested the total antifungal activities of Dicer-1-silenced termites. Our results showed that the antifungal activity was significantly decreased (Fig. 5A; t = − 3.046, df = 5, p < 0.05; paired t test) in Dicer-1-silenced termites. Furthermore, miR-71-5 was selected for further testing to determine its role in physiological defence, which was chosen according to the comparative profiling of miRNAs and mRNAs in fungus-contaminated versus naive termites and miRNA-mRNA analysis (data unpublished). We found that the antifungal activity (Fig. 5B; t = − 4.000, df = 5, p < 0.05; paired t test) was significantly reduced in termites treated with miR-71-5 simulants compared to those treated with simulant controls. These results suggested that miRNAs played an important role in driving physiological disease defences in termites.
Dicer-1 and miR-71-5 influenced the survival of fungus-contaminated termites
As both dsDicer-1 and miR-71-5 simulants led to reduced antifungal activity of termites, we tested their potential for the biological control of termites. We found that Dicer-1-silenced termites contaminated with fungus exhibited significantly decreased survival compared to that of other treated termites (dsDicer-1 + fungus vs. dsGFP + fungus: χ2 = 81.839, p < 0.001; dsDicer-1 + fungus vs dsDicer-1 + Tween 80: χ2 = 63.070, p < 0.001; dsDicer-1 + fungus vs dsGFP + Tween 80: χ2 = 65.474, p < 0.001). GFP-treated termites contaminated with fungus showed significantly decreased survival compared to the termites without fungal contamination (dsGFP + fungus versus dsDicer-1 + Tween 80: χ2 = 58.636, p < 0.001; dsGFP + fungus versus dsGFP + Tween 80: χ2 = 68.849, p < 0.001). There was no significant difference between dsDicer-1- and dsGFP-treated termites without fungal contamination (χ2 = 2.037, p = 0.154) (Fig. 6A). Additionally, the survival of fungus-contaminated termites treated with the miR-71-5 simulant was significantly decreased compared to that of other treated termites (miR-71-5 + fungus vs. control + fungus: χ2 = 63.593, p < 0.001; miR-71-5 + fungus vs. miR-71–5 + Tween 80: χ2 = 60.585, p < 0.001; miR-71-5 + fungus vs. control + Tween 80: χ2 = 67.129, p < 0.001). The survival of fungus-contaminated termites treated with the simulant control was significantly decreased compared to that of the termites without fungal contamination (control + fungus vs. miR-71-5 + Tween 80: χ2 = 61.065, p < 0.001; control + fungus vs. control + Tween 80: χ2 = 68.064, p < 0.001). There was no significant difference in survival between termites treated with the miR-71-5 simulant and simulant control without fungal contamination (miR-71-5 + Tween 80 vs. control + Tween 80: χ2 = 1.000, p = 0.317) (Fig. 6B). These results suggested a good effect of miRNAs coupled with entomopathogens in biologically controlling termites.
Effect of miRNA dysregulation on the survival of fungus-contaminated termite groups. A Survival of Dicer-1-silenced vs. GFP-treated termites with or without fungal contamination; B Survival of miR-71-5 versus control simulant-treated termites with or without fungal contamination. Different letters indicate significant differences, p < 0.05
Discussion
miRNAs perform multiple functions, such as regulating carbohydrate and energy metabolism, the immune response, oxidation–reduction reactions, and other life processes in whole bodies of the termites. As expected, ingested dsDicer-1 in termites led to Dicer-1 silencing and then reduced the miRNA concentration, suggesting the successful induction of miRNA dysregulation. Transcription analysis showed that Dicer-1 silencing was associated with 32 KEGG pathways and 175 GO terms. In social insect colonies, the salivary secretion can function as external disinfectants and operate in conjunction with social behaviour such as trophallaxis, grooming and nesting to care for offspring, nestmates and colonies (Bulmer et al. 2009; Hamilton et al. 2011; Hamilton and Bulmer 2012). The glycolysis, citrate cycle, and ATP generation from ADP are involved in carbohydrate and energy metabolism. As mounting immune responses and performing social behaviours are energetically costly, disorders of carbohydrate and energy metabolism have negative impacts on these processes (Liu et al. 2020; Hassan et al. 2021a, b; Xu et al. 2021a, b). The structural constituent of cuticle associates with insect cuticles, forming the first barrier to prevent pathogens from invading the haemocoel (Syazwan et al. 2021). Several DEGs involved in the defence response, glutathione transferase activity and oxidation–reduction process are able to encode antimicrobial peptides (AMPs), detoxification proteins, and antioxidant enzymes to increase the resistance and tolerance of insects against pathogens (Liu et al. 2015, 2019a). These defence-related functions are likely to contribute to social immunity in termite colonies. The rest of the KEGG pathways and GO terms were involved in the nervous system, digestive system, longevity, development, etc. (Supplementary material 2-Table S3 and S4). In addition, some of DEGs (e.g. carbohydrate and energy metabolism genes) in Dicer-1 silenced termites were from microbes (Supplementary material 2-Table S2), which were possibly the gut symbionts and might be important components participating in the life processes of the hosts. Therefore, miRNAs are likely to have a global impact on whole body of the termite.
Carbohydrate and energy metabolism, immune response, and oxidation–reduction reactions are important for miRNAs in driving social immunity. Animal behaviour is an energy-dependent process, and hence, some energy inhibitors cause animals to move slowly and even die (Davidson 1930; Schuler and Casida 2001). In termite colonies, disorders of glycolysis or the citrate cycle result in decreased ATP levels and inhibit locomotion behaviour (Hassan et al. 2021a, b; Xu et al. 2021a, b). Disorders of the citrate cycle also reduce the antifungal activity of termites, leading to increased infection and mortality (Liu et al. 2020). In addition, as symbionts in termite guts were reported to participate in host disease defences by providing enzyme (Rosengaus et al. 2014) and metabolites (Inagaki and Matsuura 2018), metabolic disorders in the gut symbionts were likely to reduce the symbiont-derived immunocompetence and hence diminished termite disease defences. In our study, eight metabolic genes were verified to be significantly downregulated, suggesting disorders of glycolysis and the citrate cycle in the whole bodies of Dicer-1-silenced termites. We suggested that miRNA-mediated carbohydrate and energy metabolism was an important biochemical mechanism in driving social immunity and that disruption of this process was likely to diminish behavioural and physiological disease defences in termite colonies. The insect immune system induces a large number of effector molecules to combat pathogens in vivo. Defensins are compact, cysteine-rich and protease-resistant AMPs with broad-spectrum activity against bacteria, fungi and other parasites (Weber 2021). Termicin is a termite-specific antifungal defensin whose silencing results in decreased antifungal activity and hence increased mortality of fungus-contaminated termites (Hamilton and Bulmer 2012). Insect detoxification and antioxidant systems often function in conjunction with the immune system, which effectively reduces the risk of toxins and ROS during host–pathogen interactions (Syazwan et al. 2021). Cytochrome P450 monooxygenases are responsible for catalysing the oxidation and metabolism of pathogen toxins and producing free radicals or ROS (Shankar and Mehendale 2014). The peroxiredoxin family includes antioxidative enzymes that catalyse the reduction of ROS (Xu et al. 2021a; b). Our results verified that Dicer-1 silencing significantly altered the gene expression of defensin, termicin, cytochrome P450 9e2 and peroxiredoxin-4, indicating that miRNAs played important roles in regulating the immune response and oxidation–reduction reactions in whole body of the termite. Therefore, we suggest that carbohydrate and energy metabolism, the immune response, and oxidation–reduction reactions may participate in the driving mechanism of miRNA-shaped social immunity, and their disruption may negatively impact social immunity in termite colonies.
Indeed, miRNA dysregulation has negative impacts on movement and hence behavioural defence in termite colonies. Unlike solitary insects, social insects have evolved a rich repertoire of behavioural defences, collectively detecting the presence of pathogens, limiting pathogen spread, and reducing the infection risk of individuals (Van Meyel et al. 2018; Liu et al. 2019a). Older workers perform dangerous out-of-nest tasks and are easily contaminated. When they are contaminated, nestmates tend to coalesce towards and groom contaminated workers (Liu et al. 2019b). Both contaminated workers and their caregivers increase the distance away from the rest of the naïve nestmates. Nurse workers take broods deeper into the depth of the colony to increase the distance away from the contaminated workers (Stroeymeyt et al. 2018). These results show that an increase in physical distance between colony members is an effective strategy to limit pathogen transmission [termed ‘organizational immunity’ (Liu et al. 2019a)], and locomotion should be an important social behaviour, as it adaptively adjusts the distance between contaminated individuals and naïve nestmates. Hassan and colleagues found that termites significantly enhanced their locomotion during fungal contamination, further demonstrating locomotion in response to pathogens (Hassan et al. 2021a, b). Additionally, grooming, cannibalistic and burial behaviours are well known in the management of contaminated individuals and corpses in social insect colonies. In termites contaminated with fungi, grooming behaviour occurs immediately, before germination (Liu et al. 2019b). In contrast, cannibalistic behaviour only occurs after contaminated individuals become visibly ill (Davis et al. 2018). For corpse management, fresh corpses are often regarded as nutritional rewards and induce cannibalism by producing an early death cue. In contrast, decayed corpses are regarded as infection risks and induce burial by producing late death cues (Sun et al. 2016). Here, we found that Dicer-1 silencing significantly inhibited locomotion and other movements, such as grooming, cannibalistic and burial behaviours, suggesting that miRNA dysregulation exhibited a general effect on termite movement and consequently affected behavioural defences against pathogens, pathogen-contaminated nestmates and pathogen-contaminated corpses in termite colonies. For the driving mechanism, miRNA-mediated carbohydrate and energy metabolism could be considered one of the most important biochemical factors (see the above discussion). Therefore, miRNAs could shape the behavioural defences of termites.
miRNA dysregulation also had negative impacts on the physiological defences of termites. Termites normally increase the antifungal ability by upregulation of immune genes to enhance the physiological defences (Liu et al. 2015). The gut symbiont-derived antifungal substances provide termites the ability to inhibit fungal growth to enhance the physiological defences (Rosengaus et al. 2014). Therefore, the total antifungal activity was an effective indicator of physiological disease defences of the termites. Recent studies showed that not only immune genes, but also carbohydrate metabolism and antioxidant genes were reported to be important components affecting the antifungal activity of termites (Liu et al. 2020; Zhao et al. 2020; Zhou et al. 2021). Defaunated termites were more vulnerable to fungal contamination (Rosengaus et al. 2014). Although increased the immune gene expressions such as defensin and termicin, Dicer-1-mediated miRNA dysregulation decreased the expression of carbohydrate metabolism and antioxidant genes within termites or gut symbionts and hence significantly reduced the total antifungal activity of termites. Additionally, miR-71-5 simulant-treated termites also exhibited a decrease in the total antifungal activity, further suggesting the negative impact of miRNA dysregulation on physiological defences in termites. Furthermore, the termite groups fed either dsDicer-1 or miR-71-5 simulant were more vulnerable to fungal contamination, showing an increase in infection mortality. Therefore, miRNAs could shape social immunity by driving both behavioural and physiological disease defences in termite colonies.
To our knowledge, this is the first report of the potential mechanism of miRNAs underlying social immunity in termite colonies. miRNAs can affect salivary secretion, carbohydrate and energy metabolism, cuticle generation, immune responses, oxidation–reduction reactions, etc. Among them, carbohydrate and energy metabolism is likely an important biochemical factor in driving behavioural defences such as locomotion, grooming, cannibalism, and burial in termite colonies. In addition, carbohydrate and energy metabolism, the immune response, and oxidation–reduction reactions are likely to participate in driving termite physiological defences, such as antifungal activity. Therefore, miRNA-shaped social immunity can influence the susceptibility of termite colonies to entomopathogenic fungi; hence, miRNAs may be effective targets for the biological control of termites (Fig. 7). Numerous studies have proven that RNAi-mediated silencing of target genes could lead to insect death, with considerable potential for insect pest control. In termite pest control, Zhou et al. (2008) first targeted the endoglucanase and hexamerins genes through dsRNA feeding, demonstrating the feasibility of RNAi-based termiticides. To enhance the lethal effect in termite colonies, RNAi-based termiticides coupled with infectious entomopathogenic fungi have been employed. RNAi-mediated silencing of termicin, GNBP2, TG, IDH and other coding genes coupled with Metarhizium could significantly increase the susceptibility of nestmates to socially transferred fungal conidia and hence lead to an increased infection mortality rate (Hamilton and Bulmer 2012; Liu et al. 2020; Zhao et al. 2020; Zhou et al. 2021). In our study, we demonstrated the feasibility of targeting small noncoding RNAs through feeding to optimize the biological control of termites using Metarhizium. Compared to dsRNAs specifically targeting a gene, the miRNAs were able to target a number of genes with the same or different functions, hence having a broad-spectrum impact on termite life processes. Therefore, by targeting miRNAs, termite groups exhibited diminished behavioural and physiological disease defences at both the group and individual levels, suggesting broad-spectrum inhibition of termite social immunity. By targeting miRNAs, termite groups consequently exhibited a high level of mortality during fungal contamination, suggesting that miRNAs could be employed as termiticides to enhance the biological control of termites by diminishing their social immunity. Therefore, miRNAs could be considered potential novel targets for insect pest control.
Potential mechanisms underlying social immunity in termite colonies and novel targets for biological pest control. miRNAs widely affect the whole bodies of termites, including: A carbohydrate and energy metabolism, B immune response, C oxidation–reduction reactions, etc. D Carbohydrate and energy metabolism were reported to play important roles in regulating both behaviour and antifungal activity in termites. Therefore, miRNA-mediated carbohydrate and energy metabolism was likely to be one of the genetic and biochemical factors driving behavioural (such as locomotion, grooming, cannibalism, and burial) and physiological (antifungal activity) disease defences in termite colonies. E The immune response helped termites inhibit the replication and dissemination of pathogens in the body cavity. The oxidation–reduction reaction protected termites from the damage caused by toxins and ROS from “host–pathogen” interactions. Therefore, the miRNA-mediated immune response and oxidation–reduction reaction play an important role in driving physiological disease defence in termites. F miRNAs shaped social immunity by driving behavioural and physiological disease defences in termite colonies. By targeting miRNAs, we could artificially increase the susceptibility of termite colonies to entomopathogens and consequently enhance the biological control of termites
Authors' contributions
LL, FMY, QYH and QBT conceived and designed research. LL, CCZ, and LJS conducted experiments. LL, FMY, and CCZ analysed data. LL, QYH and QBT wrote the paper. All authors read and approved the manuscript.
References
Berdnik D, Fan AP, Potter CJ, Luo L (2008) MicroRNA processing pathway regulates olfactory neuron morphogenesis. Curr Biol 18:1754–1759. https://doi.org/10.1016/j.cub.2008.09.045
Bulmer MS, Bachelet I, Raman R, Rosengaus RB, Sasisekharan R (2009) Targeting an antimicrobial effector function in insect immunity as a pest control strategy. Proc Natl Acad Sci USA 106:12652–12657. https://doi.org/10.1073/pnas.0904063106
Chouvenc T, Efstathion CA, Elliott ML, Su N-Y (2013) Extended disease resistance emerging from the faecal nest of a subterranean termite. Proc R Soc B 280:20131885. https://doi.org/10.1098/rspb.2013.1885
Davidson WM (1930) Rotenone as a contact insecticide. J Econ Entomol 23:868–874. https://doi.org/10.1093/JEE/23.5.868
Davis HE, Meconcelli S, Radek R, McMahon DP (2018) Termites shape their collective behavioural response based on stage of infection. Sci Rep 8:14433. https://doi.org/10.1038/s41598-018-32721-7
Esparza-Mora MA, Davis HE, Meconcelli S, Plarre R, McMahon DP (2020) Inhibition of a secreted immune molecule interferes with termite social immunity. Front Ecol Evol 8:75. https://doi.org/10.3389/fevo.2020.00075
Gomez-Orte E, Belles X (2009) MicroRNA-dependent metamorphosis in hemimetabolan insects. Proc Natl Acad Sci USA 106:21678–21682. https://doi.org/10.1073/pnas.0907391106
Hamilton C, Bulmer MS (2012) Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Dev Comp Immunol 36:372–377. https://doi.org/10.1016/j.dci.2011.07.008
Hamilton C, Lejeune BT, Rosengaus RB (2011) Trophallaxis and prophylaxis: social immunity in the carpenter ant Camponotus pennsylvanicus. Biol Lett 7:89–92. https://doi.org/10.1098/rsbl.2010.0466
Hassan A, Huang Q, Mehmood N, Xu H, Zhou W, Gao Y (2021a) Alteration of termite locomotion and allogrooming in response to infection by pathogenic fungi. J Econ Entomol 114:1256–1263. https://doi.org/10.1093/jee/toab071
Hassan A, Huang Q, Xu H, Wu J, Mehmood N (2021b) Silencing of the phosphofructokinase gene impairs glycolysis and causes abnormal locomotion in the subterranean termite Reticulitermes chinensis Snyder. Insect Mol Biol 30:57–70. https://doi.org/10.1111/imb.12672
He S et al (2018) Termite soldiers contribute to social immunity by synthesizing potent oral secretions. Insect Mol Biol 27:564–576. https://doi.org/10.1111/imb.12499
Hong M, Hwang D, Cho S (2018) Hemocyte Morphology and cellular immune response in termite (Reticulitermes speratus). J Insect Sci 18:46. https://doi.org/10.1093/jisesa/iey039
Hussain A, Li Y-F, Cheng Y, Liu Y, Chen C-C, Wen S-Y (2013) Immune-related transcriptome of Coptotermes formosanus Shiraki workers: the defense mechanism. PLoS ONE 8:e69543. https://doi.org/10.1371/journal.pone.0069543
Inagaki T, Matsuura K (2018) Extended mutualism between termites and gut microbes: nutritional symbionts contribute to nest hygiene. Sci Nat 105:52. https://doi.org/10.1007/s00114-018-1580-y
Kumar S, Upadhyay RK (2021) Anti-termite potential of various bio-organic constituents with special reference to family asteraceae. WJPR 10:1109–1149. https://doi.org/10.20959/wjpr20213-19977
Lee YS et al (2004) Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117:69–81. https://doi.org/10.1016/S0092-8674(04)00261-2
Liu L, Li G-H, Sun P-D, Lei C-L, Huang Q-Y (2015) Experimental verification and molecular basis of active immunization against fungal pathogens in termites. Sci Rep 5:15106. https://doi.org/10.1038/srep15106
Liu L, Wang W, Liu Y-L, Sun P-D, Lei C-L, Huang Q-Y (2019a) The influence of allogrooming behavior on individual innate immunity in the subterranean termite Reticulitermes chinensis (Isoptera: Rhinotermitidae). J Insect Sci 19:6. https://doi.org/10.1093/jisesa/iey119
Liu L, Zhao X-Y, Tang Q-B, Lei C-L, Huang Q-Y (2019b) The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 11:244. https://doi.org/10.3390/toxins11050244
Liu L, Wang C-C, Zhao X-Y, Guan J-X, Lei C-L, Huang Q-Y (2020) Isocitrate dehydrogenase-mediated metabolic disorders disrupt active immunization against fungal pathogens in eusocial termites. J Pest Sci 93:291–301. https://doi.org/10.1007/s10340-019-01164-y
Lopez-Uribe MM, Sconiers WB, Frank SD, Dunn RR, Tarpy DR (2016) Reduced cellular immune response in social insect lineages. Biol Lett 12:20150984. https://doi.org/10.1098/rsbl.2015.0984
Lucas KJ, Zhao B, Liu S, Raikhel AS (2015) Regulation of physiological processes by microRNAs in insects. Curr Opin Insect Sci 11:1–7. https://doi.org/10.1016/j.cois.2015.06.004
Rosengaus RB, Jordan C, Lefebvre ML, Traniello JFA (1999) Pathogen alarm behavior in a termite: A new form of communication in social insects. Naturwissenschaften 86:544–548. https://doi.org/10.1007/s001140050672
Rosengaus RB, Lefebvre ML, Traniello JFA (2000) Inhibition of fungal spore germination by nasutitermes: evidence for a possible antiseptic role of soldier defensive secretions. J Chem Ecol 26:21–39. https://doi.org/10.1023/A:1005481209579
Rosengaus RB, Schultheis KF, Yalonetskaya A, Bulmer MS, DuComb WS, Benson RW, Thottam JP, Godoy-Carter V (2014) Symbiont-derived β-1,3-glucanases in a social insect: mutualism beyond nutrition. Front Microbiol 5:607. https://doi.org/10.3389/fmicb.2014.00607
Schuler F, Casida JE (2001) The insecticide target in the PSST subunit of complex I. Pest Manag Sci 57:932–940. https://doi.org/10.1002/ps.364
Seipke RF et al (2011) A single Streptomyces symbiont makes multiple antifungals to support the fungus farming ant Acromyrmex octospinosus. PLoS ONE 6:e22028. https://doi.org/10.1371/journal.pone.0022028
Shankar K, Mehendale HM (2014) Cytochrome P450. In: Wexler P (ed) Encyclopedia of toxicology. Academic Press, pp 1125–1127
Stroeymeyt N, Grasse AV, Crespi A, Mersch DP, Cremer S, Keller L (2018) Social network plasticity decreases disease transmission in a eusocial insect. Science 362:941–945. https://doi.org/10.1126/science.aat4793
Sun Q, Haynes KF, Zhou XG (2016) Dynamic changes in death cues modulate risks and rewards of corpse management in a social insect. Funct Ecol 31:697–706. https://doi.org/10.1111/1365-2435.12754
Syazwan SA, Lee SY, Sajap AS, Lau WH, Omar D, Mohamed R (2021) Interaction between Metarhizium anisopliae and its host, the subterranean termite Coptotermes curvignathus during the infection process. Biology 10:263. https://doi.org/10.3390/biology10040263
Terrapon N et al (2014) Molecular traces of alternative social organization in a termite genome. Nat Commun 5:3636. https://doi.org/10.1038/ncomms4636
Van Meyel S, Körner M, Meunier J (2018) Social immunity: why we should study its nature, evolution and functions across all social systems. Curr Opin Insect Sci 28:1–7. https://doi.org/10.1016/j.cois.2018.03.004
Verma M, Sharma S, Prasad R (2009) Biological alternatives for termite control: a review. Int Biodeter Biodegr 63:959–972. https://doi.org/10.1016/j.ibiod.2009.05.009
Wang YL, Yang ML, Jiang F, Zhang JZ, Kang L (2013) MicroRNA-dependent development revealed by RNA interference-mediated gene silencing of LmDicer1 in the migratory locust. Insect Sci 20:53–60. https://doi.org/10.1111/j.1744-7917.2012.01542.x
Weber F (2021) Antiviral innate immunity: introduction. In: Bamford DH, Zuckerman M (eds) Encyclopedia of virology. Elsevier, pp 577–583
Xu H, Huang Q-Y, Gao Y-Y, Wu J, Hassan A, Liu Y-T (2021a) IDH knockdown alters foraging behavior in the termite odontotermes formosanus in different social contexts. Curr Zool 67:609. https://doi.org/10.1093/cz/zoab032
Xu Z, Zeng X, Li M, Liao J, Chen Q (2021b) MicroRNA-383 promotes reactive oxygen species-induced autophagy via downregulating peroxiredoxin 3 in human glioma U87 cells. Exp Ther Med 21:439. https://doi.org/10.3892/etm.2021.9870
Yanagawa A, Fujiwara-Tsujii N, Akino T, Yoshimura T, Yanagawa T, Shimizu S (2011) Musty odor of entomopathogens enhances disease-prevention behaviors in the termite Coptotermes formosanus. J Invertebr Pathol 108:1–6. https://doi.org/10.1016/j.jip.2011.06.001
Yanagawa A, Imai T, Akino T, Toh Y, Yoshimura T (2015) Olfactory cues from pathogenic fungus affect the direction of motion of termites, Coptotermes formosanus. J Chem Ecol 41:1118–1126. https://doi.org/10.1007/s10886-015-0649-8
Yang ML et al (2014) MicroRNA-133 inhibits behavioral aggregation by controlling dopamine synthesis in locusts. PLoS Genet 10:e1004206. https://doi.org/10.1371/journal.pgen.1004206
Zhang S et al (2018) Host miRNAs are involved in hormonal regulation of HaSNPV-triggered climbing behaviour in Helicoverpa armigera. Mol Ecol 27:459–475. https://doi.org/10.1111/mec.14457
Zhao X-Y, Liu L, Zhou W, Cai Q, Huang Q-Y (2020) Roles of selenoprotein T and transglutaminase in active immunization against entomopathogenic fungi in the termite Reticulitermes chinensis. J Insect Physiol 125:104085. https://doi.org/10.1016/j.jinsphys.2020.104085
Zhou X, Wheeler MM, Oi FM, Scharf ME (2008) RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem Mol Biol 38:805–815. https://doi.org/10.1016/j.ibmb.2008.05.005
Zhou W, Huang Q-Y, Zhao X-Y, Liu L, Mehmood N (2021) Silencing of selenium-binding protein disrupted the active immunization of the termite Reticulitermes chinensis and improved the lethal effect of the entomopathogenic fungus Metarhizium anisopliae. Biol Control 157:104588. https://doi.org/10.1016/j.biocontrol.2021.104588
Acknowledgements
We thank Dong-Huai Wang, Long-Long Sun, Jia-Jia Zhang, and Yun-Liang Du for collecting termite samples in the field. We thank Mian Faisal Nazir and Nasir Mehmood for correcting the manuscript. We also thank and other group members in the Department of Entomology for the help during our experiments.
Funding
This work was supported by the Science and Technology Planning Project of Henan Province of China (Grant No. 212102110140) and the National Natural Science Foundation of China (Grant Nos. 31672367 and 31572322).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Subba Reddy Palli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Video S1 Video of locomotion in termites between Dicer-1-silenced and GFP-treated termites (MP4 5453 KB)
Video S2 Video of grooming in termite groups between Dicer-1-silenced and GFP-treated termites (MP4 4849 KB)
Video S3 Video of cannibalism in termite groups between Dicer-1-silenced and GFP-treated termites (MP4 18867 KB)
Video S4 Video of burial in termite groups between Dicer-1-silenced and GFP-treated termites (MP4 18865 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, L., Yan, FM., Zhao, CC. et al. microRNAs shape social immunity: a potential target for biological control of the termite Reticulitermes chinensis. J Pest Sci 96, 265–279 (2023). https://doi.org/10.1007/s10340-022-01495-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-022-01495-3