Abstract
Up to 75% of nitrogen (N) taken up during cauliflowers production is allocated to leaves, which are left as crop residues after harvest. The inclusion of cauliflower, cultivated alone or intercropped with legumes, in rotation schemes, is a promising tool to optimize N availability to subsequent crops. This original study assessed, for the first time in South Tyrol, Italy, the effect of removal or soil incorporation of cauliflower and clover residues on the growth and N uptake of subsequent lettuce. In 2015, cauliflower was sole-cropped or intercropped with clover, under different N regimes (N0, N1, N2, N3 = 0, 75, 150, 300 kg N ha−1). Cauliflower and clover residues were either removed or incorporated in the soil in 2016. The effects of the residual fertility left by the N fertilizer, the two cropping systems, and the crop residues were assessed on the yield and N uptake of two subsequent lettuce crops. Isotopic 15N-labeled cauliflower residues were additionally used to quantify the N contribution of cauliflower residues to lettuce growth. During the first lettuce crop, residues incorporation was the only factor increasing lettuce yields (+41%) and N uptake (+58%). The residual effect of N1 and N2 rates increased the lettuce N uptake when clover residues were incorporated. During the second lettuce crop, residues incorporation increased lettuce yields (+26%) and N uptake (+44%). On average, 64% and 35% of the lettuce N amounts, in the first and second cycles, respectively, derived from cauliflower residues, and accounted for 38% of the total N contained in cauliflower residues (214 kg N ha−1). Results from this experiment, uncommon for the examined species, demonstrate that incorporation of cauliflower and clover residues provides an excellent source of N for lettuce. Incorporating residues of the preceding cauliflower crop, alone or intercropped with clover, before establishing the lettuce crop, substantially reduce the N fertilization needs of subsequent lettuce crops.
Similar content being viewed by others
1 Introduction
Vegetable crops, and particularly brassica species, are often associated with low nitrogen (N) use efficiencies, high N demand, and large N losses (Castellanos et al. 2001; Agneessens et al. 2014). Cauliflower (Brassica oleracea var. botrytis (L.) Alef.) is one of the most important brassica crops cultivated worldwide (FAO 2020) and, together with broccoli, is grown on over 1.35 M ha and produces about 25.7 M tons of marketable yield (average 2015–2019; FAO 2021). The amount of N contained in the brassica residues at harvest can exceed 200 kg N ha−1 (Rahn et al. 1992) and in cauliflower can contain 50–75% of total N taken up by the crop (Thompson et al. 2000; Li 2011; Tempesta et al. 2019b). Such significant N amounts represent, if properly managed, an important source of N for subsequent crops (De Neve and Hofman 1998; Cameron et al. 2013).
Xie and Kristensen (2016) intercropped cauliflower with clover and proved that the combination of a legume intercrop under suboptimal N fertilization could limit the nitrate losses whilst maintaining optimal cauliflower yields. The inclusion of a legume cover crop in intercropping systems, subsequently converted into mulch, better supports water management as well as a higher availability of mineral nutrients in the soil due to the mineralization of the mulch residues (Campiglia et al. 2011). Crop residues left on the field after harvesting can enhance N availability and are beneficial for the successive crop (Rochester et al. 2001; Li et al. 2020). A recent study (Fontaine et al. 2020) on the residual N effect by clover used as cover crop showed that clover residues contain around 50 kg N ha−1, suggesting that the inclusion of legumes in rotation systems is a promising technique to enhance the soil N availability thanks to the biological N fixation (BNF) of the rhizobia associated with the legumes. To which extent the soil can benefit from the BNF also depends on the soil N available for the clover (Tempesta et al. 2019b). The BNF rate progressively decreases at increasing N fertilizer rates (Tempesta et al. 2019a).
The amount of N released from crop residues that can potentially be taken up by following crops largely depends on N mineralization and immobilization processes by soil microbial biomass (Chen et al. 2014). One of the most important factors that can affect N release from residues is the C:N ratio, which affects the mineralization-immobilization balance, but also the forms of carbon and nitrogen play a crucial role on microbial activity and subsequent decomposition of residues (Abiven et al. 2005). If soil mineral N is limited for microorganisms, a low C:N ratio due to a high N content in residues generally increases the microbial activity enhancing the rate of decomposition, whereas a high C:N ratio causes N immobilization (Kumar and Goh 2002; Lemtiri et al. 2016). Otherwise, the biochemical characteristics of the residues may have a major contribution in the regulation of microbial activity and subsequent decomposition (Chaves et al. 2021): plant residues, with high N concentrations, low lignin, and cellulose concentrations (low C:N and lignin:N ratios) often result in high N mineralization rates (Chaves et al. 2004; Manzoni et al. 2008; Gentile et al. 2009). In crop residues, total high N content (low C:N) is usually also inversely related to high soluble residue content, which impacts degradation rate (Redin et al. 2014). Therefore, in legume-based intercropping and rotation systems, the C:N ratio of crop residues is generally low, and their mineralization rate high, resulting in an increase of both yield and N uptake of the succeeding crop (Li et al. 2020; Fontaine et al. 2020). Being able to predict the amount and dynamics of release of the residue N would allow fine tuning of the N supply to the following crop, with a significant N fertilizer reduction. Such knowledge is available for some rotation systems like potato-cauliflower (Akkal-Corfini et al. 2010) or potato-maize (Duenas et al. 2005), but few studies have been conducted on how efficiently the N derived from cauliflower residues is utilized by subsequent vegetable crops (Nett et al. 2010).
This experiment was part of a 3-year innovative study (2015–2017) carried out for the first time in South Tyrol, an alpine region of Northern Italy, focused on the optimization of N inputs by means of fertilizer management, intercropping systems, and crop residue recycling in crop rotations. The first experiment aimed at assessing the effect of both increasing N fertilizer rates and the presence or absence of a clover intercrop (Trifolium resupinatum L. var. Gorby) on cauliflower (Brassica oleracea var. botrytis (L) Alef, cv Seoul F1, Vilmorin) growth, yield, and N uptake (Tempesta et al. 2019b). In addition, the potential contribution of N from the symbiotic fixation of clover was investigated (Tempesta et al. 2019a).
The second experiment lasted from October 2015, after the cauliflower harvest, to October 2016, and assessed the effect of different cauliflower and clover residue management (removal or soil incorporation) on the growth and N uptake of two subsequent lettuce crop cycles. The suitability of lettuce crop to be used as an indicator of fertilizer residual effects is well known (Gianquinto and Borin 1996). We hypothesized (1) that returning cauliflower aboveground residues to soil contributes to meeting the N needs of lettuce (Lactuca sativa var. capitata (L.) Janchen) grown in rotation; (2) that intercropping cauliflower and clover results in a greater amount of biomass and of residual N to be taken up by the lettuce crop; and (3) that the effects of the fertilizer N applied to the preceding crop are still apparent on successive lettuce crops.
2 Material and methods
2.1 Preceding intercropping trial
Successive crop rotation trials were undertaken at Laimburg Research Center experimental farm in Oris/Eyrs (Bolzano/Bozen, Venosta Valley, South Tyrol — Italy, 46°37′23.2″ N, 10°38′53.4″ E, 900 m.a.s.l). A 2-year experiment (2015–2016; figures 1 and 2) was carried out on cauliflower following potato (Solanum tuberosum L.), cultivated from April to September 2014, and winter oat (Avena sativa) as a cover crop to prevent soil erosion, from September 2014 to March 2015. In this region, cauliflower is the most common Brassica species grown in rotation with lettuce (Lactuca sativa L.), potato (Solanum tuberosum L.), broccoli (Brassica oleracea var. Italica), or onion (Allium cepa L.).
Experimental setup: (a) sole-cropped cauliflower and (b) cauliflower intercropped with clover in spring 2015; (c) aboveground crop residues removed (left) or not (right), in winter 2015–2016; (d) lettuce crop in 2016; (e) first lettuce yield in 2016, with visible size difference between lettuce heads harvested in sub-sub-plots where aboveground crop residues were removed (left) or not (right). Photographs by Marco Tempesta.
Chronology of the main actions carried out during the experiments on cauliflower and clover crops in 2015 (top part of the chart) and on lettuce crops in 2016 (bottom part of the chart). The lettuce crops in 2016 did not receive any N fertilization. The N fertilizer was supplied only in 2015 to both cauliflower and clover crops.
During the first year, the experiment considered four increasing rates of N fertilizer (N0, N1, N2, N3 = 0, 75, 150, 300 kg N ha−1) supplied to two cropping systems (CS): sole cauliflower (S) and cauliflower intercropped with clover (IC). The experimental design was a 4 × 2 split-plot with three blocks. The main plot factor was assigned to the N rate (N factor) while the sub-plot factor to the CS (CS factor), S or IC (experimental unit = 9.5 m2; Fig. 1a,b). Cauliflower was transplanted at the end of June 2015, in rows 0.65 m × 0.42 m apart (3.6 plants m−2) and annual clover was sown at a rate of 2.5 g of seeds per m2 in order to obtain a uniform cover between the cauliflower rows (Fig. 2). The N fertilizer, ammonium nitrate (NH4NO3; 27% N), was supplied as top dressing and equally split at two growth stages, 20 days after transplanting (vegetative stage) and 20 days before harvest (reproductive stage) (Fig. 2).
The cauliflower harvest took place in September 2015, 80 days after transplanting, when yield, crop-residues biomass of cauliflower and clover, and their N content were assessed (Fig. 2). Further details on the experiment layout and management, data collection and analysis, and results of this trial are reported in Tempesta et al. (2019b).
2.2 Lettuce growth cycles
On October 20, 2015, after cauliflower curd harvest, each sub-plot was divided into two equal parts (sub-sub-plots): aboveground residues of both cauliflower (leaves) and clover (leaves and stems) were either manually removed from the soil in one sub-sub-plot, or mechanically chopped (using a stalk shredder) and left on top of the soil surface of the other sub–sub-plot (Fig. 1c). Each experimental unit had an area of approximately 5 m2.
Soil analyses were conducted for mineral N concentration both after cauliflower harvest (October 2015) and prior to lettuce transplanting (May 2016) (Fig. 2). Soil chemical and physical properties were assessed in April 2016 prior to lettuce transplanting (Table 1). Meteorological data (Min–Max temperature = −9.3 °C in January and 23 °C in June; Min–Max rainfall = 0 mm in December and 105 mm in June) were recorded through a weather station placed in the research facility (Fig. S1 - Supplementary material).
On 17 May 2016, the upper 0.3 m soil layer of the entire plot was mechanically tilled (disk harrowing) and the crop residues of cauliflower and clover, whose biomass and N content were previously assessed, were incorporated into the top 0.2 m layer. In 2016, two consecutive cultivation cycles of Iceberg lettuce were carried out (Fig. 2) to assess the growth and N uptake of the lettuce relative to (a) the residual fertilizer nitrogen supplied in 2015; (b) the presence or absence of intercropped clover in the preceding crop cycle; (c) the incorporation or the removal of aboveground crop residues. No N fertilizer was supplied to the lettuce crops in 2016. The experiment was set as a split-split-plot 4 × 2 × 2 factorial design with 3 blocks having the following structure: (1) the main plot factor (N factor): residual effect of four nitrogen levels applied in 2015 (N0, N1, N2, N3 = 0, 75, 150, 300 kg N ha−1); 2) the sub-plot factor (CS factor): residual effect of two crop levels in 2015 (S and IC); (3) the sub-sub-plot factor (residues or R factor): two crop residues levels (R+ = aboveground residues left, R− = aboveground residues removed).
On 15 April 2016, Iceberg lettuce cv. Umbrinas (Rijk Zwaan Zaadteelt en Zaadhandel B.V.) was sown in trays with a mixture of peat and vermiculite and placed in a greenhouse until 20 May 2016. Seedlings were transplanted at a planting distance of 0.32 m between rows and 0.35 m within row, for a total of 20 plants per experimental unit (plant density = 8.9 plants m−2). Manual weeding was carried out twice during the lettuce crop cycle, which lasted 55 days from transplanting to harvest. On 13 July 2016, the lettuce was harvested by cutting the head at soil level and removing the outermost leaves (Fig. 1d, e).
The aboveground crop residues left after the first lettuce harvest were removed from the field, and the soil was disk harrowed. Lettuce plants of the same cultivar were transplanted on 25 July 2016, at the same planting density. Manual weeding was carried out twice during the crop cycle that lasted 56 days from transplanting to harvest. At harvest time, the lettuce heads were harvested and the aboveground biomass was sampled.
2.2.1 Lettuce uptake of N derived from labeled cauliflower residues
A complementary experiment was nested inside the main experiment (Fig. 2), with the aim of quantifying the amount of N derived from the decomposition of cauliflower leaves and taken up by lettuce plants. In October 2015, a portion of the original cauliflower leaf residue (approximately 60% of the total, corresponding to 300 g m−2 dry weight = DW), present in the plots where cauliflower was sole cropped and fertilized with N1, were substituted with the same amount of 15N-labeled cauliflower residues. To obtain labeled cauliflower residues, sixty cauliflower seedlings were grown outdoor from 13 July 2015 (transplanting date) until 20 October 2015 (harvest date) in pots filled with a mix of soil and vermiculite (diameter 18.5 cm, 24 cm depth) and received a nutrient solution containing 15N-enriched ammonium nitrate (15NH415NO3, 10.0 at. % enrichment) split in four different steps, one application every 15 days (200 ml of nutrient solution per plant). On 20 October 2015, cauliflower leaves were collected and mixed with the unlabeled cauliflower leaves, and both were left on top of the labeled plots in the field. A representative sample was collected and analyzed to determine the N concentration and 15N abundance.
2.3 Sampling, analysis, and calculations
Soil analyses for mineral N concentration determination were performed using the following methodology. Four soil cores were collected with a 30-mm-diameter auger (Eijkelkamp, NL) from 0 to 40 cm soil layer of each experimental unit and their nitrate and ammonium concentration determined. The collected soil samples were homogenized and a fresh 10-g subsample was oven dried at 65°C and weighed. Each soil subsample was transferred in a 500-ml flask and mixed with 100 ml of 2M KCl. Flasks were shaken on a horizontal shaker for 1 h. The supernatant was collected, filtered, transferred into a 50 ml tube, and centrifuged for 10 min at 3000 rpm. The analysis of ammonium and nitrate concentration was performed by a continuous flow autoanalyzer (AA-3; Bran+Luebbe, Norderstedt, Germany).
Fresh weight (FW) of crop residues of cauliflower and clover were assessed in each experimental unit after cauliflower harvest. The aboveground organs where harvested and roots collected from 0 to 40 cm soil layer after sieving. A subsample of each plant organ of both cauliflower and clover was fresh weighed, dried at 60 °C up to constant weight, dry weighed, and analyzed for total N and carbon (C) concentration with an elemental analyzer (Flash EA 2000, ThermoFisher Scientific). The C:N ratio of both types of residues (cauliflower alone and cauliflower plus clover) was calculated. Total crop residues N content was calculated as the sum of the product of dry biomass of each plant organ (leaves, roots) by its N concentration.
In each lettuce cycles, a sample of three plants was collected from the center of sub-plot, then leaves were separated, the soil was sieved, and roots collected. The FW of lettuce leaves and roots was assessed and the marketable yield quantified. Sub-samples (100 g per plant) of fresh leaves and roots of each plant were oven dried at 60 °C to a constant dry weight (DW), milled, and analyzed for total N concentration with an elemental analyzer (Flash EA 2000, ThermoFisher Scientific). Lettuce N uptake was calculated as the sum of the product of root and leaf dry biomass by their N concentration.
Leaves and roots of lettuce grown in the labeled plots were also analyzed with an elemental analyzer (Flash EA 2000, ThermoFisher Scientific) coupled with an isotope ratio mass spectrometer (Delta V Advantage, ThermoFisher Scientific) in order to obtain their N concentration and their 15N abundance. The 15N atomic abundance values of both cauliflower residues and lettuce leaves and roots were transformed into the corresponding 15N atomic excess values, after subtracting the 15N atomic natural abundance, obtained from extra lettuce plants, not exposed to the labeled N residues.
The N derived from labeled cauliflower residues (Ndflr%), the lettuce uptake of N from labeled residues (Nuflr), and the N-recovery were calculated according to Zapata (1990), using the following formulas:
2.4 Data analysis
Data about cauliflower and clover crop residue biomass and their N content were analyzed by a split-plot ANOVA (Gomez and Gomez 1984; Mendiburu 2017) with two factors. The main plot factor was assigned to the N rate (N factor) while the sub-plot factor to the cropping system (CS factor). Data collected from the rotation experiment were subjected to a split-split-plot ANOVA with three factors: N factor (main plot factor), CS factor (sub-plot factor), and R factor (sub-sub-plot factor). Means were separated using Tukey HSD test (Hothorn et al. 2008) at p≤0.05. Before the analysis, all data were checked for normality and homogeneity of the variance. Averages and standard errors (SE) were calculated. Statistical analysis was carried out using R statistical software version 3.3.2 (R Core Team 2016).
3 Results
3.1 Cauliflower and clover residues
Results of soil mineral N obtained from both analyses conducted after the cauliflower harvest and prior to lettuce transplanting did not differ, indicating a negligible N leaching due to scarce precipitation and the presence of mainly snow on the field during the winter (data not shown). In addition, the increasing rates of N supplied to the cauliflower crop did not significantly affect the soil mineral N present at transplanting of the lettuce the following year (Tempesta et al. 2019b) that, nonetheless, resulted to be relatively low (2.9–5.5 mg mineral N per kg dry soil). Regardless of CS, most biomass and N content were present in the aboveground residues, as it appears from the comparison between the amounts recorded in R+ and R− plots (Table 2). In R+ where S preceded the lettuce, crop residues had a biomass range of 765–935 g m−2 (DW) and contained some 25–27 g N m−2 (Table 2). The total residue biomass left by IC in R+ instead, ranged 775–1290 g m−2 (DW) and showed a peak with N1. Moreover, with N1 and N2 fertilizer rates, IC had a significantly (p ≤ 0.05) higher amount of N (37 g N m−2 and 33 g N m−2, for N1 and N2 respectively) compared to S (Table 2). The C:N ratio of IC was not affected by N level (Table 2). Crop residues of S had a C:N ratio ranging 14–25 in R− and 12–13 in R+, while that of residues derived from IC (weighted average of the C:N from both cauliflower and clover) ranged 16–22 and 11–16 in R− and R+, respectively (Table 2). The C:N ratio of only clover (data not shown) varied between 15 and 17 in R− and from 18 to 24 in R+.
3.2 Biomass, yield, and N content of lettuce
The root DW of lettuce plants sampled at the end of the first production cycle was significantly affected by the R factor (p ≤ 0.01), but not by the residual N fertilizer and CS (Table 3). The root DW was higher in R+ than in R− (9.5 g m−2 versus 7.2 g m−2), although it represented only the 4% of the total lettuce DW.
In the first lettuce crop, leaf biomass was affected by CS (p ≤ 0.05), R (p ≤ 0.001), and a significant interaction (p ≤ 0.01) between R and N rates was observed (Table 3). The N rates had no effect on leaf DW in R−, while a significant DW increase was observed where N supply had been combined with crop residues incorporation (R+). With the exception of N0, leaf DW was always significantly higher in R+ than in R−. Leaf biomass was always higher in IC than in S (Table 3). The marketable yields were affected by both CS (p ≤ 0.05) and R (p ≤ 0.001), and by the interaction (p ≤ 0.01) between R and the residual N rates (Table 3). Irrespective of N rates and R, lettuce yield was higher in IC compared to S. In addition, lettuce yield was always higher in R+ compared to R−. The residual N from fertilizer did not affect the lettuce yield in R−, but did so in R+ where the highest lettuce yields were obtained at N2 rate, and the lowest at N0 rate (Table 3).
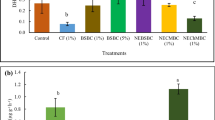
Leaf N content accounted for 97% of total lettuce N (root + leaf) in both R+ and R−. Total lettuce N content was strongly affected by CS (p ≤ 0.01), by R (p ≤ 0.001), and by the interaction between R and N rates (p ≤ 0.01), as well by the interaction among the three factors (p ≤ 0.05). In general, plants contained more N when grown in R+ than in R− (Fig. 3).
First lettuce crop: interaction effect among N rates (residual fertilizer; N0, N1, N2, N3 = 0, 75, 150, 300 kg N ha−1), CS (residual cropping systems), and R (removal or incorporation of aboveground residues) on total N content (root + leaf) of lettuce during the first cycle. Black and grey bars refer to sole cauliflower (S) or intercropping cauliflower with clover (IC) in the previous cropping system, respectively. Vertical lines above the bars represent the standard error. Lower case letters indicate statistical differences among the N rates within each level of R and CS, and capital letters indicate differences between the two CS within each N rate for each level of R and N (Tukey HSD test at p ≤ 0.05).
Total N content in R− was unaffected by the N rate, but in N0 lettuce accumulated more N when it followed IC (Fig. 3). In R+ and when lettuce was cultivated after S, the total plant N content was unaffected by the N rate. However, when lettuce succeeded IC, lettuce N uptake progressively increased from N0 to N2. This result follows the trend observed for the N content of the crop residues assessed after the cauliflower curd harvest (Table 2).
Yields, leaf biomass and plant N content at the end of the second cycle were significantly affected by the R factor only (p ≤ 0.001). They were higher in R+ compared to R− (Table 4). The biomass of lettuce plants decreased, on average, by 19% in R− and by 25% in R+, as compared to the first cycle, while the total N uptake decreased by 9% and 17% in R− and R+, respectively.
3.3 N uptake from labeled cauliflower residues
The soil incorporation of labeled cauliflower residues (only in N1, R+) allowed an accurate assessment of the N derived from cauliflower residues taken up by lettuce plants. The total N amount in the residues incorporated into the soil was 21.4 g N m−2 and their average 15N abundance was 0.79 at. % 15N. The15N abundance of lettuce leaves harvested after the first and the second production cycle was 0.67±0.04 and 0.52±0.01 at. % 15N, respectively. The N derived from labeled cauliflower residues (Ndflr), accounted therefore for 64% and 35% of total N in lettuce plants. Considering both production cycles together, lettuce plants took up 9.80 g of N m−2 deriving from cauliflower residues (Nuflr, Table 5), representing 46% of the total N contained in labeled cauliflower residues (N-Recovery, Table 5).
4 Discussion
The incorporation of crop residues, the preceding crop system (sole cauliflower or intercropping cauliflower + clover), and the N fertilizer rate applied to the preceding crop had significant effects on the subsequent lettuce cycles (Fig. 4).
Main N fluxes and climatic conditions during the experiments in 2015 and 2016. The chart shows fluxes of N absorbed, left in the soil, and recycled by crop residues in sole-cropped cauliflower plot, fertilized with N1 rate. The white sections of the bars on the left correspond to the growing seasons.
The incorporation of the aboveground residues had the largest effect on yields and N uptake by lettuce (+41% and +51%, respectively, for lettuce grown during the first crop cycle). Such effects were robust and durable, as they were still visible when the second lettuce cycle was grown (+ 26% and + 44% for yield and leaf N content, respectively). According to our experiment with 15N-enriched aboveground residues, cauliflowers represent an excellent source of N for the following crops. Indeed, within 16 weeks after cauliflower residue incorporation, two subsequent lettuce cycles recovered almost 100 kg N ha−1 (about 70 and 30 kg N ha−1 in the first and second lettuce crop, respectively). The recovery of N from cauliflower (46% in total) resulted to be in line with the literature, indicating a short-term mineralization rate of 40–60% of the N from cauliflower residues (De Neve and Hofman 1998; Guerette et al. 2002; Akkal-Corfini et al. 2010).
The quality of the residues probably played an important role on their mineralization rate. Interestingly, the whole plant cauliflower residues had a C:N ratio of 12–13, regardless of the N level and in line with Chaves et al. (2007) (cauliflower leaf C:N ratio 9.78–10.3; cauliflower leaf + stems C:N ratio 11.8), and cauliflower roots residues had a C:N ratio ranging 14–25, quite similar to that reported by Chaves et al. (2004) for cabbages roots. The average C:N ratio of residues left after the intercropping system (weighted average of the C:N from both cauliflower and clover) ranged 16 to 22 in roots, while for the aboveground residues ranged 11 to 16. This difference was probably due to a different content of lignin, a fact that might have influenced their decomposition (Vigil and Kissel 1991; Cabrera et al. 2005) and the behavior of the following lettuce crop, as a result of the inverse relationship between the decay rate of crop residues and their C:N ratio. Literature reports that in legume-based intercropping and rotation systems, the C:N ratio of crop residues is generally low, and their mineralization rate high, resulting in an increase of both yield and N uptake of the succeeding crop (e.g., Li et al. 2020; Fontaine et al. 2020). This effect on the succeeding crop was also observed in our study, mainly during the first lettuce cycle where the presence of clover promoted growth and yield of lettuce plants (+17% yield, on average) and enhanced the N uptake in some N fertilizer rates. This result confirms that clover residues are a readily available N source for the following crop.
The differences in C:N ratio of mixture residues components were also evidenced in our first-year experiment (Tempesta et al. 2019b), where cauliflower roots had higher C:N ratio as compared to leaves while opposite trend was observed in clover. Furthermore, clover roots had lower C:N ratio than cauliflower roots, while the opposite occurred for leaves (Tempesta et al. 2019b). We can speculate that whenever clover and cauliflower residues are mixed (only roots or whole plant residues) C:N ratio of mixture remains quite low and balanced by the presence of a low C:N ratio component (e.g., clover roots or cauliflower leaves). Accordingly, when the residues are composed only by roots (R−), the lower C:N ratio of clover roots stimulates the microbial activity, fostering the decomposition of clover root residues but also cauliflower root residues. In the present experiment, this was confirmed by the fact that clover had a positive effect on leaf biomass and yield of lettuce when aboveground residues were removed (R−) thanks to differences in decomposition rates between the cauliflower and clover roots left in the soil (Redin et al. 2014; Chaves et al. 2021), as well as some other improved soil conditions due to the legume such as an enhanced soil water retention, a greater aggregate stability (McVay et al. 1989) or a significant reduction of soil nitrogen losses (Drinkwater et al. 1998). On the other hand, when also the aboveground portion of plant are incorporated into the soil (R+), the low C:N ratio of cauliflower leaves promote the decomposition of both crop residues. Anyhow, the C:N ratio of residues measured in our experiment was lower than the critical C:N ratio (30:1) as stated by Probert et al. (2005) below which N net mineralization occurs fostering N availability for subsequent crops. This relatively low C:N ratio coupled with the low soil mineral content reported by Tempesta et al. (2019b) (2.9–5.5 mg mineral N per kg dry soil) could have promoted the microbial activity enhancing the rate of residues decomposition (Kumar and Goh 2002; Lemtiri et al. 2016; Chaves et al. 2021), and accordingly N availability for successive cropping cycles.
The increasing rates of N supplied to the preceding cauliflower crop, although not resulting in different residual soil mineral N, affected growth and yield of the following lettuce crop. Such an effect was observed only when the aboveground residues were incorporated into the soil and during the first crop cycle. This effect can be attributed to the presence of most of the residual N in the aboveground organs of the preceding crop and to the fact that the N rates significantly affected the amount of N in the residues. The crop residues had a biomass ranging about 770–1300 g m−2 (DW) and contained some 25–37 g N m−2. These amounts of N are higher than those reported by Everaarts et al. (1996) (10–12 g N m−2), but similar to Kage et al. (2002) (900 g m−2 DW, 30 g N m−2), who analyzed the dry matter and N partitioning in cauliflower, grown in a typical loess-derived hapludalf soil, following a wide range of N supply.
Besides the contribution to the nutrition of subsequent crop cycle, another important aspect related to the use of crop residues is the synchronization between N release and N uptake (Chen et al. 2014). In our experiments, no differences were observed in the concentration of soil mineral N performed at the end of cauliflower growing cycle (October 2015) and at the beginning of the first lettuce growing cycle (May 2016). We can speculate that the environmental factors played an important role on the rate of mineralization of the organic N contained in the plant residues. Indeed, between October 2015 and March 2016, air temperature remained lower than 10 °C and precipitation was mostly snowy (Fig. 4; Fig. S1 - Supplementary material), factors that combined can be the cause of an arrest in N mineralization (Wu and McGechan 1998). The higher temperature and the rainfall occurred before and during the first growing cycle of lettuce (from April 2016 until the end of July 2016) led to a faster net mineralization of the crop residues, as suggested by Quemada and Cabrera (1997) and Thapa et al. (2021), which confirmed a robust impact of soil moisture and air temperature on N net mineralization of crop residues. The environmental parameter patterns (e.g., air temperature and precipitations) observed in the present experiment can be considered as representative of the average climate of the considered area (Italian Alpine environment), generally characterized by cold and snowy winter, and warm and wet summer. Considering the absence of differences in soil mineral N concentration between the end of cauliflower crop cycle and the beginning of lettuce crop cycle, it can be advanced that the climate pattern during fall and winter was unfavorable to microbial activity, which was then fostered in spring by higher temperature and presence of rain, enabling a synchronization between the N availability in soil due to mineralization and the beginning of the lettuce crop cycle. On the other hand, in regions where warmer and wetter fall and winter may promote microbial activity with a consequent enhanced risk of nitrate leaching through the soil, the use of a cover crop can be useful to reduce N loss and to recycle mineral N for crop in rotation.
As stated before, the two subsequent lettuce cycles recovered almost 100 kg N ha−1 from the cauliflower residues. This amount of N fulfills a significant part of the N needs of lettuce crops and allows to limit the supply of N fertilizers, in line with the national guidelines on good agricultural practices, which suggest not to exceed 110 kg N ha−1 in the lettuce crop, to avoid the risk of nitrate accumulation in the leaves (MIPAAF 2021; Santamaria et al. 2013). However, the 46% total N-recovery of the two lettuce cycles suggests that the N contained in crop residues was not totally released during the season. The remaining N could have been lost, immobilized by the microbial population or still present in the original organic matter during its transformation process to humus. It is therefore speculated that additional cropping cycles on the same soil could benefit from the residual N contained in the crop residues.
5 Conclusion
Cauliflower residues proved to be an excellent N source and an efficient contributor for enhancing lettuce yields cultivated in succession contributing almost one half of its N requirement. The presence of clover intercropped with cauliflower has an additional positive effect on the lettuce grown in succession, and its inclusion in a rotation scheme has the potential to further reduce the lettuce N fertilizer needs. Thanks to the innovative results shown in this paper, vegetable growers might consider the proposed rotation scheme (cauliflower-clover followed by lettuce or another N demanding vegetable crops) and adjust their lettuce fertilization plan (cutting off amounts of N similar to 100 kg N ha−1) thanks to the incorporation of crop residues. This study brings an original contribution to the existing literature on both 15N application on vegetable crops and on quantification of N derived from the decomposition of cauliflower residues. Results contained in this manuscript, relatively rare for the species considered, deliver new, practical, and useful information on N fertilization management and crop rotations to the farmers of Alpine regions.
Data availability
All significant data generated or analyzed during this study are included in this published article except for the soil mineral N data (non-significant results), which are available in Tempesta et al. (2019b). Statistically, non-significant results which have not been shown are available from the corresponding author on reasonable request.
References
Abiven S, Recous S, Reyes V, Oliver R (2005) Mineralisation of C and N from root, stem and leaf residues in soil and role of their biochemical quality. Biol Fertil Soils 422(42):119–128. https://doi.org/10.1007/S00374-005-0006-0
Agneessens L, De Waele J, De Neve S et al (2014) Review of alternative management options of vegetable crop residues to reduce nitrate leaching in intensive vegetable rotations. Agronomy 4:529–555. https://doi.org/10.3390/agronomy4040529
Akkal-Corfini N, Morvan T, Menasseri-Aubry S, Bissuel-Bélaygue C, Poulain D, Orsini F, Leterme P (2010) Nitrogen mineralization, plant uptake and nitrate leaching following the incorporation of (15N)-labeled cauliflower crop residues (Brassica oleracea) into the soil: a 3-year lysimeter study. Plant Soil 328:17–26
Cabrera ML, Kissel DE, Vigil MF (2005) Nitrogen mineralization from organic residues. J Environ Qual 34:75–79. https://doi.org/10.2134/jeq2005.0075
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann. Appl. Biol. 162:145–173
Campiglia E, Mancinelli R, Radicetti E (2011) Influence of no-tillage and organic mulching on tomato (Solanum Lycopersicum L.) production and nitrogen use in the Mediterranean environment of central Italy. Sci Hortic (Amsterdam) 130:588–598. https://doi.org/10.1016/j.scienta.2011.08.012
Castellanos JZ, Villalobos S, Delgado JA, Muñoz-Ramos J, Sosa A, Vargas P, Lazcano I, Alvarez-Sanchez E, Enriquez SA (2001) Use of best management practices to increase nitrogen use efficiency and protect environmental quality in a broccoli-corn rotation of central Mexico. Commun Soil Sci Plant Anal 32:1265–1292. https://doi.org/10.1081/CSS-100104112
Chaves B, De Neve S, Hofman G et al (2004) Nitrogen mineralization of vegetable root residues and green manures as related to their (bio)chemical composition. Eur J Agron 21:161–170. https://doi.org/10.1016/j.eja.2003.07.001
Chaves B, De Neve S, Piulats LM et al (2007) Manipulating the N release from N-rich crop residues by using organic wastes on soils with different textures. Soil Use Manag 23:212–219. https://doi.org/10.1111/j.1475-2743.2006.00063.x
Chaves B, Redin M, Giacomini SJ, Schmatz R, Léonard J, Ferchaud F, Recous S (2021) The combination of residue quality, residue placement and soil mineral N content drives C and N dynamics by modifying N availability to microbial decomposers. Soil Biol Biochem 163:108434. https://doi.org/10.1016/J.SOILBIO.2021.108434
Chen B, Liu E, Tian Q, Yan C, Zhang Y (2014) Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 34:429–442
De Neve S, Hofman G (1998) N mineralization and nitrate leaching from vegetable crop residues under field conditions: a model evaluation. Soil Biol Biochem 30:2067–2075. https://doi.org/10.1016/S0038-0717(98)00082-0
Drinkwater LE, Wagoner P, Sarrantonio M (1998) Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 396:262–265. https://doi.org/10.1038/24376
Duenas G, Muniz O, Lopez T, Zapata F (2005) Nitrogen recycling in a potato-maize-potato sequence. In: In: International Atomic Energy Agency. IAEA Fusion Energy Conference (20th : 2004 : Vilamoura, Portugal). Published by International Atomic Energy Agency
Everaarts AP, De Moel CP, Van Noordwijk M (1996) The effect of nitrogen and the method of application on nitrogen uptake of cauliflower and on nitrogen in crop residues and soil at harvest. Netherlands J Agric Sci 44:43–55. https://doi.org/10.18174/njas.v44i1.557
FAO (2020) Fruit and vegetables – your dietary essentials. FAO, Rome. https://doi.org/10.4060/cb2395en
FAO (2021) Food and Agriculture Organization of the United Nations. FAOSTAT statistical database. http://www.fao.org/faostat/en/#data/QC
Fontaine D, Eriksen J, Sørensen P (2020) Cover crop and cereal straw management influence the residual nitrogen effect. Eur J Agron 118:126100. https://doi.org/10.1016/j.eja.2020.126100
Gentile R, Vanlauwe B, van Kessel C, Six J (2009) Managing N availability and losses by combining fertilizer-N with different quality residues in Kenya. Agric Ecosyst Environ 131:308–314. https://doi.org/10.1016/J.AGEE.2009.02.003
Gianquinto G, Borin M (1996) Quality response of crisphead lettuce and kohlrabi to mineral and organic fertilization in different soils. Adv Hortic Sci:20–28. https://doi.org/10.1400/75285
Gomez KA, Gomez AA (1984) Statistical Procedure for Agricultural Research, Second Edi edn. John Wiley and Sons, New York
Guerette V, Desjardins Y, Belec C et al (2002) Nitrogen contribution from mineralization of vegetable crop residues. Acta Hortic 571:95–102. https://doi.org/10.17660/ActaHortic.2002.571.10
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J. 50:346–363
Kage H, Alt C, Stützel H (2002) Nitrogen concentration of cauliflower organs as determined by organ size, N supply, and radiation environment. Plant Soil 246:201–209. https://doi.org/10.1023/A:1020627723616
Kumar K, Goh KM (2002) Management practices of antecedent leguminous and non-leguminous crop residues in relation to winter wheat yields, nitrogen uptake, soil nitrogen mineralization and simple nitrogen balance. Eur J Agron 16:295–308. https://doi.org/10.1016/S1161-0301(01)00133-2
Lemtiri A, Degrune F, Barbieux S et al (2016) Gestion des résidus de cultures dans les systèmes de grandes cultures sous climat tempéré. Partie 1: Propriétés biologique et chimique (phosphore et azote) du sol (synthèse bibliographique). Biotechnol. Agron. Soc. Environ. 20:236–244
Li H (2011) Cauliflower plant organ water status and nitrogen translocation from leaf-stem sources to head sinks in its three hybrid cultivars. Int J Veg Sci 17:363–382. https://doi.org/10.1080/19315260.2011.565113
Li F, Sørensen P, Li X, Olesen JE (2020) Carbon and nitrogen mineralization differ between incorporated shoots and roots of legume versus non-legume based cover crops. Plant Soil 446:243–257. https://doi.org/10.1007/s11104-019-04358-6
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science (80- ) 321:684–686. https://doi.org/10.1126/SCIENCE.1159792/SUPPL_FILE/MANZONI.SOM.PDF
McVay KA, Radcliffe DE, Hargrove WL (1989) Winter legume effects on soil properties and nitrogen fertilizer requirements. Soil Sci Soc Am J 53:1856–1862. https://doi.org/10.2136/sssaj1989.03615995005300060040x
Mendiburu F (2017) Agricolae: statistical procedures for agricultural research. In: R package version 1.2-8. R. Foundation for Statistical Computing, Vienna, AUS
MIPAAF (2021) Linee guida nazionali di produzione integrata 2021/sezione tecniche agronomiche Rev. 5 del 12 11 2020. . https://www.reterurale.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/22126.
Nett L, Feller C, Fink M (2010) Identification of crop rotations including nitrogen catch crops which can decrease nitrogen losses in German field vegetable production systems. In: In: Acta Horticulturae, pp 239–246
Probert ME, Delve RJ, Kimani SK, Dimes JP (2005) Modelling nitrogen mineralization from manures: representing quality aspects by varying C:N ratio of sub-pools. Soil Biol Biochem 37:279–287. https://doi.org/10.1016/J.SOILBIO.2004.07.040
Quemada M, Cabrera ML (1997) Temperature and moisture effects on C and N mineralization from surface applied clover residue. Plant Soil 189:127–137. https://doi.org/10.1023/A:1004281804058
R Core Team (2016) R: a language and environment for statistical computing. www.R-project.org/
Rahn CR, Vaidyanathan LV, Paterson CD (1992) Nitrogen residues from brassica crops. Asp Appl Biol 30:263–270
Redin M, Recous S, Aita C, Dietrich G, Skolaude AC, Ludke WH, Schmatz R, Giacomini SJ (2014) How the chemical composition and heterogeneity of crop residue mixtures decomposing at the soil surface affects C and N mineralization. Soil Biol Biochem 78:65–75. https://doi.org/10.1016/J.SOILBIO.2014.07.014
Rochester IJ, Peoples MB, Hulugalle NR, Gault RR, Constable GA (2001) Using legumes to enhance nitrogen fertility and improve soil condition in cotton cropping systems. F Crop Res 70:27–41. https://doi.org/10.1016/S0378-4290(00)00151-9
Santamaria P, Gonnella M, Di Gioia F, et al. (2013) LATTUGA. In: Incrocci L, Dimauro B, Santamaria P, Pardossi A (eds) La concimazione azotata degli ortaggi. pp 121–139
Tempesta M, Gianquinto G, Hauser M, Tagliavini M (2019a) Potential nitrogen contribution from symbiotic fixation of dwarf pea (Pisum sativum) and clover (Trifolium resupinatum) in crop rotation and intercropping systems. In: Horticulturae A (ed) International Society for Horticultural Science (ISHS), Leuven, Belgium, pp 261–266
Tempesta M, Gianquinto G, Hauser M, Tagliavini M (2019b) Optimization of nitrogen nutrition of cauliflower intercropped with clover and in rotation with lettuce. Sci Hortic (Amsterdam) 246:734–740. https://doi.org/10.1016/j.scienta.2018.11.020
Thapa R, Tully KL, Cabrera ML, Dann C, Schomberg HH, Timlin D, Reberg-Horton C, Gaskin J, Davis BW, Mirsky SB (2021) Effects of moisture and temperature on C and N mineralization from surface-applied cover crop residues. Biol Fertil Soils 57:485–498. https://doi.org/10.1007/s00374-021-01543-7
Thompson TL, Doerge TA, Godin RE (2000) Nitrogen and water interactions in subsurface drip-irrigated cauliflower II. Agronomic, Economic, and Environmental Outcomes. Soil Sci Soc Am J 64:412–418. https://doi.org/10.2136/sssaj2000.641412x
Vigil MF, Kissel DE (1991) Equations for estimating the amount of nitrogen mineralized from crop residues. Soil Sci Soc Am J 55:757–761. https://doi.org/10.2136/sssaj1991.03615995005500030020x
Wu L, McGechan MB (1998) A review of carbon and nitrogen processes in four soil nitrogen dynamics models. J Agric Eng Res 69:279–305. https://doi.org/10.1006/JAER.1997.0250
Xie Y, Kristensen HL (2016) Overwintering grass-clover as intercrop and moderately reduced nitrogen fertilization maintain yield and reduce the risk of nitrate leaching in an organic cauliflower (Brassica oleracea L. var. botrytis) agroecosystem. Sci Hortic (Amsterdam) 206:71–79. https://doi.org/10.1016/j.scienta.2016.04.034
Zapata F (1990) Isotope techniques in soil fertility and plant nutrition studies. In: Hardarson G (ed) Training course series: No. 2. International Atomic Energy Agency, Vienna, pp 61–127
Acknowledgements
We acknowledge the technical support of the Laimburg Research Centre of Vegetable Crops for their help in the fieldwork. Special thanks are extended to Martina Frapporti and Christian Grumer for their precious help during the soil and plant sampling. We are grateful to Christian Ceccon and Valentina Lucchetta from the Free University of Bozen-Bolzano for their help in laboratory analysis. We thank Pr Nick Rahiri Roskruge, Massey University, Palmerston North (New Zealand) for proofreading the article, and we would like to thank the anonymous reviewers and the editor in chief Dr. Sylvie Recous for their valuable suggestions and comments, which led to substantial improvements of this paper.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE.
Author information
Authors and Affiliations
Contributions
The study conception and design, material preparation, data collection, and analysis were performed by Marco Tempesta, Giorgio Gianquinto, Markus Hauser, and Massimo Tagliavini. The first draft of the manuscript was written by Marco Tempesta and all authors, included Dr. Giuseppina Pennisi, contributed to improve the quality of the manuscript. All authors read and approved the final manuscript and its submission to your journal.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The origiinal online version of this article was revised: Missing Open Access funding information has been added in the Funding Note.
Supplementary Information
ESM 1
(DOCX 75 kb)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tempesta, M., Pennisi, G., Gianquinto, G. et al. Contribution of cauliflower residues to N nutrition of subsequent lettuce crops grown in rotation in an Italian Alpine environment. Agron. Sustain. Dev. 42, 25 (2022). https://doi.org/10.1007/s13593-022-00756-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-022-00756-w