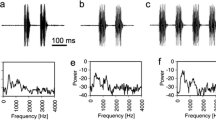
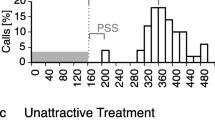
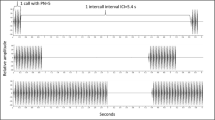
Abstract
In anuran amphibians, as well as many other animals, it is common for males to form breeding aggregations where they advertise to females of the same species. For female gray treefrogs (Hyla versicolor), the characteristics of male calls are integral to their preferences while the distance to the males represents a key feature of the required investment. Therefore, the spatial arrangement of males and the quality of their advertisement calls can influence sexual selection processes. We recorded the calls of male gray treefrogs, as well as the spatial position of individuals in the chorus, for aggregations in northern Michigan over three breeding seasons. Males were not randomly distributed across the chorus but showed both clustered patterns and dispersed patterns, depending on the scale of analysis. On active chorus nights, we identified clusters of males and individual males closest to the centers of those clusters (“medoids”) using a novel analytical approach. Medoids displayed some call characteristics that are preferred by females significantly more often than the other members of their clusters and thus may represent “hotshot” males. Non-medoid males in the clusters displayed less preferred call features. Irrespective of overall cluster membership, the size of the chorus (number of males calling per night) and nearest neighbor distances were also correlated with specific call features. We suggest that the smaller clusters within choruses that we identified may represent the spatial scale over which female gray treefrogs sample and choose male mates.
Significance statement
Aggregations of animals for the sole purpose of mating are common and conspicuous occurrences. However, such aggregations provide both costs and benefits to participants. For males, the quality of their displays and spatial location in the aggregation can determine whether they mate at all. Females also face constraints as their preferred mates may represent costly choices. Using an objective point pattern analysis technique, we demonstrated that the male advertisement choruses of the gray treefrog can be subdivided into smaller local groups that contain higher quality males at the center. These local groups display some features of classic “hotshot” style leks and may represent the units females consider for choice of male mates.







Similar content being viewed by others
Data availability
The data set for this study has been uploaded to CurateND https://curate.nd.edu/ and can be accessed via https://doi.org/10.7274/r0-h2g2-gq69.
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Arak A (1988) Female mate selection in the natterjack toad - active choice or passive attraction. Behav Ecol Sociobiol 22:317–327
Baddeley A, Rubak E, Turner R (2016) Spatial point patterns: methodology and applications with R. Chapman and Hall/CRC Press, London
Balmford A (1991) Mate choice on leks. Trends Ecol Evol 6:87–92
Bates ME, Cropp BF, Gonchar M, Knowles J, Simmons JA, Simmons AM (2010) Spatial location influences vocal interactions in bullfrog choruses. J Acoust Soc Am 127:2664–2677
Bee MA (2008) Parallel female preferences for call duration in a diploid ancestor of an allotetraploid treefrog. Anim Behav 76:845–853
Bee MA (2012) Sound source perception in anuran amphibians. Curr Opin Neurobiol 22:301–310
Bee MA, Reichert MS, Tumulty J (2016) Assessment and recognition of rivals in anuran contests. Adv Stud Behav 48:161–249
Beehler BM, Foster MS (1988) Hotshots, hotspots, and female preference in the organization of lek mating systems. Am Nat 131:203–219
Bernal XE, Rand AS, Ryan MJ (2007) Sexual differences in the behavioral response of tungara frogs, Physalaemus pustulosus, to cues associated with increased predation risk. Ethology 113:755–763
Bertram S, Berrill M, Nol E (1996) Male mating success and variation in chorus attendance within and among breeding seasons in the gray treefrog (Hyla versicolor). Copeia 1996:729–734
Bevier CR (1997) Utilization of energy substrates during calling activity in tropical frogs. Behav Ecol Sociobiol 41:343–352
Bonachea LA, Ryan MJ (2011) Localization error and search costs during mate choice in Tungara frogs, Physalaemus pustulosus. Ethology 117:56–62
Bourne GR (1992) Lekking behavior in the neotropical frog Ololygon rubra. Behav Ecol Sociobiol 31:173–180
Bowyer RT, McCullough DR, Rachlow JL, Ciuti S, Whiting JC (2020) Evolution of ungulate mating systems: Integrating social and environmental factors. Ecol Evol 10:5160–5178
Boyd SK, Gordon NM (2021) Auditory and distance cues interact to modulate female gray treefrog preferences for male advertisement calls. Behav Ecol Sociobiol 75:95
Boyko AR, Gibson RM, Lucas JR (2004) How predation risk affects the temporal dynamics of avian leks: Greater sage grouse versus golden eagles. Am Nat 163:154–165
Bradbury J, Gibson R (1983) Leks and mate choice. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 109–138
Bradbury J, Gibson R, Tsai IM (1986) Hotspots and the dispersion of leks. Anim Behav 34:1694–1709
Brenowitz EA (1989) Neighbor call amplitude influences aggressive behavior and intermale spacing in choruses of the Pacific treefrog (Hyla regilla). Ethology 83:69–79
Brenowitz EA, Rose GJ (1994) Behavioral plasticity mediates aggression in choruses of the Pacific treefrog. Anim Behav 47:633–641
Brenowitz EA, Wilczynski W, Zakon HH (1984) Acoustic communication in spring peepers – Environmental and behavioral aspects. J Comp Physiol 155:585–592
Brockmann HJ, St Mary CM, Ponciano JM (2018) Discovering structural complexity and its causes: Breeding aggregations in horseshoe crabs. Anim Behav 143:177–191
Burmeister S, Konieczka J, Wilczynski W (1999) Agonistic encounters in a cricket frog (Acris crepitans) chorus: Behavioral outcomes vary with local competition and within the breeding season. Ethology 105:335–347
Bush SL, Gerhardt HC, Schul J (2002) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14
Deb R, Balakrishnan R (2014) The opportunity for sampling: the ecological context of female mate choice. Behav Ecol 25:967–974
Diggle P (2003) The statistical analysis of spatial point patterns, 2nd edn. Hodder Education Publishers, London
Doty GV, Welch AM (2001) Advertisement call duration indicates good genes for offspring feeding rate in gray tree frogs (Hyla versicolor). Behav Ecol Sociobiol 49:150–156
Duraes R, Loiselle BA, Parker PG, Blake JG (2009) Female mate choice across spatial scales: influence of lek and male attributes on mating success of blue-crowned manakins. Proc R Soc Lond B 276:1875–1881
Evans JC, Votier SC, Dall SRX (2016) Information use in colonial living. Biol Rev 91:658–672
Fellers GM (1979a) Aggression, territoriality, and mating behavior in North American treefrogs. Anim Behav 27:107–119
Fellers GM (1979b) Mate selection in the gray treefrog, Hyla versicolor. Copeia 1979:286–290
Forester DC, Lykens DV (1986) Significance of satellite males in a population of spring peepers (Hyla crucifer) Copeia. 1986:719–724
Forsman A, Hagman M (2006) Calling is an honest indicator of paternal genetic quality in poison frogs. Evolution 60:2148–2157
Gayou DC (1984) Effects of temperature on the mating call of Hyla versicolor. Copeia 1984:733–738
Gerhardt HC (1978) Temperature coupling in the vocal communication system of the gray tree frog, Hyla versicolor. Science 199:992–994
Gerhardt HC, Daniel RE, Perrill SA, Schramm S (1987) Mating behavior and male mating success in the green treefrog. Anim Behav 35:1490–1503
Gerhardt HC, Diekamp B, Ptacek M (1989) Inter-male spacing in choruses of the spring peeper, Pseudacris (Hyla) crucifer. Anim Behav 38:1012–1024
Gerhardt HC, Dyson ML, Tanner SD (1996) Dynamic properties of the advertisement calls of gray tree frogs: Patterns of variability and female choice. Behav Ecol 7:7–18
Gerhardt HC, Klump GM (1988) Masking of acoustic signals by the chorus background noise in the green tree frog - a limitation on mate choice. Anim Behav 36:1247–1249
Gerhardt HC, Tanner SD, Corrigan CM, Walton HC (2000) Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav Ecol 11:663–669
Gibson RM (1996) Female choice in sage grouse: The roles of attraction and active comparison. Behav Ecol Sociobiol 39:55–59
Girgenrath M, Marsh RL (1997) In vivo performance of trunk muscles in tree frogs during calling. J Exp Biol 200:3101–3108
Glos J, Wegner F, Dausmann KH, Linsenmair KE (2008) Oviposition site selection in an endangered Madagascan frog: Experimental evaluation of a habitat model and its implications for conservation. Biotropica 40:646–652
Godwin GJ, Roble SM (1983) Mating success in male treefrogs, Hyla chrysoscelis (Anura, Hylidae). Herpetologica 39:141–146
Grafe U (1997) Use of metabolic substrates in the gray treefrog Hyla versicolor: Implications for calling behavior. Copeia 1997:356–362
Greenfield MD, Rand AS (2000) Frogs have rules: Selective attention algorithms regulate chorusing in Physalaemus pustulosus (Leptodactylidae). Ethology 106:331–347
Han J, Kamber M, Pei J (2012) Data Mining: Concepts and Techniques, 3rd edn. Morgan Kaufmann Publishers Inc, San Francisco
Höbel G, Barta T (2014) Adaptive plasticity in calling site selection in grey treefrogs (Hyla versicolor). Behaviour 151:741–754
Hoglund J, Alatalo RV (1995) Leks. Princeton University Press, Princeton
Jain AK (2010) Data clustering: 50 years beyond K-means. Pattern Recogn Lett 31:651–666
Janetos AC (1980) Strategies of female mate choice - a theoretical analysis. Behav Ecol Sociobiol 7:107–112
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev 72:283–327
Jiguet F, Arroyo B, Bretagnolle V (2000) Lek mating systems: a case study in the little bustard Tetrax tetrax. Behav Process 51:63–82
Johnson JR, Semlitsch RD (2003) Defining core habitat of local populations of the gray treefrog (Hyla versicolor) based on choice of oviposition site. Oecologia 137:205–210
Jones TM, Quinnell RJ, Balmford A (1998) Fisherian flies: benefits of female choice in a lekking sandfly. Proc R Soc Lond B 265:1651–1657
Kassambara A, Mundt F (2017) factoextra: Extract and visualize the results of multivariate data analyses. R package version 1.0.5, https://CRAN.R-project.org/package=factoextra
Kaufmann L, Rousseeuw PJ (1987) Clustering by means of medoids. In: Dodge Y (ed) Statistical Data Analysis Based on the L1 Norm. Elsevier, Amsterdam, pp 405–416
Kaufman L, Rousseeuw PJ (1990) Finding Groups in Data: An Introduction to Cluster Analysis. John Wiley & Sons, New York
Klump GM, Gerhardt HC (1987) Use of non-arbitrary acoustic criteria in mate choice by female gray tree frogs. Nature 326:286–288
Kohler J, Jansen M, Rodriguez A, Kok PJR, Toledo LF, Emmrich M, Glaw F, Haddad CFB, Rodel MO, Vences M (2017) The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa 4251:1–124
Lopez PT, Narins PM, Lewis ER, Moore SW (1988) Acoustically induced call modification in the white-lipped frog, Leptodactylus albilabris. Anim Behav 36:1295–1308
Macedo RH, Podos J, Graves JA, Manica LT (2018) Breeding clusters in birds: ecological selective contexts, mating systems and the role of extrapair fertilizations. Anim Behav 143:145–154
Mackenzie A, Reynolds JD, Brown VJ, Sutherland WJ (1995) Variation in male mating success on leks. Am Nat 145:633–652
Maechler M, Rousseeuw PJ, Struyf A, Hubert M, Hornik K (2021) cluster: Cluster analysis basics and extensions. R package version 2.1.1, https://CRAN.R-project.org/package=cluster
Manica LT, Graves JA, Podos J, Macedo RH (2020) Hidden leks in a migratory songbird: mating advantages for earlier and more attractive males. Behav Ecol 31:1180–1191
McLister JD (2001) Physical factors affecting the cost and efficiency of sound production in the treefrog Hyla versicolor. J Exp Biol 204:69–80
McLister JD, Stevens ED, Bogart JP (1995) Comparative contractile dynamics of calling and locomotor muslces in 3 hylid frogs. J Exp Biol 198:1527–1538
Meuche I, Brusa O, Linsenmair KE, Keller A, Prohl H (2013) Only distance matters - non-choosy females in a poison frog population. Front Zool 10:16
Morris MR (1989) Female choice of large males in the treefrog Hyla chrysoscelis - the importance of identifying the scale of choice. Behav Ecol Sociobiol 25:275–281
Naguib M, Wiley RH (2001) Estimating the distance to a source of sound: mechanisms and adaptations for long-range communication. Anim Behav 62:825–837
Nekola JC, Kraft CE (2002) Spatial constraint of peatland butterfly occurrences within a heterogeneous landscape. Oecologia 130:53–61
Ovaska K, Hunte W (1992) Male mating behavior of the frog Eleutherodactylus johnstonei (Leptodactylidae) in Barbados, West Indies. Herpetologica 48:40–49
Perez DM, Backwell PRY (2019) Male spacing and female choice in a fiddler crab. Behav Ecol 30:1769–1774
Ptacek MB (1992) Calling sites used by male gray treefrogs, Hyla versicolor and Hyla chrysoscelis, in sympatry and allopatry in Missouri. Herpetologica 48:373–382
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/
Real L (1990) Search theory and mate choice.1. Models of Single-Sex Discrimination. Am Nat 136:376–405
Reichert MS, Gerhardt HC (2013a) Gray tree frogs, Hyla versicolor, give lower-frequency aggressive calls in more escalated contests. Behav Ecol Sociobiol 67:795–804
Reichert MS, Gerhardt HC (2013b) Socially mediated plasticity in call timing in the gray tree frog, Hyla versicolor. Behav Ecol 24:393–401
Reichert MS, Hobel G (2018) Phenotypic integration and the evolution of signal repertoires: a case study of treefrog acoustic communication. Ecol Evol 8:3410–3429
Richardson C, Lena JP, Joly P, Lengagne T (2008) Are leaders good mates? A study of call timing and male quality in a chorus situation. Anim Behav 76:1487–1495
Ringler M, Szipl G, Hodl W, Khil L, Kofler B, Lonauer M, Provin C, Ringler E (2017) Acoustic ranging in poison frogs–it is not about signal amplitude alone. Behav Ecol Sociobiol 71:114
Ripley BD (1977) Modeling spatial patterns. J Roy Stat Soc B Met 39:172–212
Robertson JGM (1984) Acoustic spacing by breeding males of Uperoleia rugosa (Anura, leptodactylidae). J Compar Ethol 64:283–297
Robertson JGM (1986) Male teritoriality, fighting and assessment of fighting ability in the Australian frog, Uperoleia rugosa. Anim Behav 34:763–772
Rousseeuw PJ (1987) Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20:53–65
Rudolf VHW, Rodel MO (2005) Oviposition site selection in a complex and variable environment: the role of habitat quality and conspecific cues. Oecologia 142:316–325
Ryan MJ, Tuttle MD, Taft LK (1981) The costs and benefits of frog chorusing behavior. Behav Ecol Sociobiol 8:273–278
Schubert E, Rousseeuw PJ (2019) Faster k-medoids clustering: Improving the PAM, CLARA, and CLARANS algorithms. In: Amato G, Gennaro C, Oria V, Radovanović M (eds) Similarity Search and Applications. SISAP 2019. Lecture Notes in Computer Science, vol 11807. Springer International Publishing, Cham, pp 171–187
Schwartz JJ (1987) The function of call alternation in anuran amphibians: A test of three hypotheses. Evolution 41:461–471
Schwartz JJ (1993) Male calling behavior, female discrimination and acoustic interference in the neotropical treefrog Hyla microcephala under realistic acoustic conditions. Behav Ecol Sociobiol 32:401–414
Schwartz JJ, Buchanan BW, Gerhardt HC (2001) Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol 49:443–455
Schwartz JJ, Buchanan BW, Gerhardt HC (2002) Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav Ecol Sociobiol 53:9–19
Schwartz JJ, Gerhardt HC (1989) Spatially mediated release from auditory masking in an anuran amphibian. J Comp Physiol A 166:37–41
Schwartz JJ, Hunce R, Lentine B, Powers K (2016) Calling site choice and its impact on call degradation and call attractiveness in the gray treefrog, Hyla versicolor. Behav Ecol Sociobiol 70:1–19
Stewart MM, Bishop PJ (1994) Effects of increased sound level of advertisement calls on calling male frogs, Eleuthrodactylus coqui. J Herpetol 28:46–53
Stillman RA, Clutton-Brock TH, Sutherland WJ (1993) Black holes, mate retention, and the evolution of ungulate leks. Behav Ecol 4:1–6
Stratman KD, Oldehoeft EA, Hobel G (2021) Woe is the loner: Female treefrogs prefer clusters of displaying males over single “hotshot” males. Evolution 75:3026–3036
Sullivan BK, Hinshaw SH (1992) Female choice and selection on male calling behavior in the gray treefrog Hyla versicolor. Anim Behav 44:733–744
Tárano Z (2009) Structure of transient vocal assemblages of Physalaemus fischeri (Anura, Leiuperidae): Calling site fidelity and spatial distribution of males. S Am J Herpetol 4:43–50
Tárano Z, Fuenmayor E (2013) Mate choice based on acoustic features in Johnstone’s whistling frog Eleutherodactylus johnstonei: an experimental approach. S Am J Herpetol 8:52–59
Tarof SA, Ratcliffe LM, Kasumovic MM, Boag PT (2005) Are least flycatcher (Empidonax minimus) clusters hidden leks? Behav Ecol 16:207–217
Tejedo M (1992) Large male mating advantage in natterjack toads, Bufo calamita - Sexual selection or energetic constraints. Anim Behav 44:557–569
Telford SR (1985) Mechanisms and evolution of inter-male spacing in the painted reedfrog (Hyperolius marmoratus). Anim Behav 33:1353–1361
Telford SR, Dyson ML, Passmore NI (1989) Mate choice occurs only in small choruses of painted reed frogs Hyperolius marmoratus. Bioacoustics 2:47–53
Wagner WE, Sullivan BK (1992) Chorus organization in the Gulf Coast toad (Bufo valliceps) - Male and female behavior and the opportunity for sexual selection. Copeia 1992:647–658
Welch AM (2003) Genetic benefits of a female mating preference in gray tree frogs are context-dependent. Evolution 57:883–893
Welch AM, Semlitsch RD, Gerhardt HC (1998) Call duration as an indicator of genetic quality in male gray tree frogs. Science 280:1928–1930
Wells KD (1977) The social behavior of anuran amphibians. Anim Behav 25:666–693
Wells KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago
Wells KD, Schwartz JJ (1984) Vocal communication in a neotropical treefrog, Hyla ebraccata - Advertisement calls. Anim Behav 32:405–420
Wells KD, Taigen TL (1986) The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19:9–18
Westcott DA (1994) Leks of leks - A role for hotspots in lek evolution. Proc R Soc Lond B 258:281–286
Whitney CL, Krebs JR (1975) Spacing and calling in Pacific treefrogs, Hyla regilla. Can J Zool 53:1519–1527
Wilczynski W, Brenowitz EA (1988) Acoustic cues mediate inter-male spacing in a neotropical frog. Anim Behav 36:1054–1063
Woodward B (1982) Male persistence and mating success in Woodhouse’s toad (Bufo woodhousei). Ecology 63:583–585
Acknowledgements
The authors gratefully acknowledge the helpful comments of the editors and reviewers.
Funding
The authors gratefully acknowledge the support of the National Science Foundation, https://www.nsf.gov/ (IOS #1257777 to SKB). We are also grateful to the University of Notre Dame Environmental Research Center, https://underc.nd.edu/, for providing facilities and financial support for this project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable guidelines for the care and use of animals were followed. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Notre Dame. Animals were used under a Michigan Department of Natural Resources scientific collector’s permit.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by K. Summers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leverett, M.C., McLister, J.D., Desaivre, S.S. et al. Social modulation of spatial dynamics in treefrog choruses. Behav Ecol Sociobiol 76, 54 (2022). https://doi.org/10.1007/s00265-022-03163-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03163-z