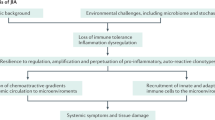
Abstract
The classification of inflammatory arthritis incorporates a sharp divide between diseases of childhood onset, grouped together as juvenile idiopathic arthritis, and diseases such as rheumatoid arthritis that begin by definition in adulthood. An important consequence of this divide is that regulatory authorities and many rheumatologists regard pediatric and adult arthritides as truly different, with the implication that drugs should be evaluated separately for each category. However, it is now clear that most forms of arthritis transcend the pediatric/adult boundary and that agents generally exhibit comparable success irrespective of age of onset, offering new opportunities in drug development and regulation focused on pharmacology and safety rather than efficacy. This paradigm shift will enable advances in arthritis treatment, originating either with adults or children, to translate more rapidly across the age spectrum.

Similar content being viewed by others
References
Ansell BM, Bywaters EG. Prognosis in Still’s disease. Bull Rheum Dis. 1959;9(9):189–92.
Bywaters EG. Heberden oration, 1966. Categorization in medicine: a survey of Still’s disease. Ann Rheum Dis. 1967;26(3):185–93.
Ansell BM. Heberden Oration, 1977. Chronic arthritis in childhood. Ann Rheum Dis. 1978;37(2):107–20.
Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Clin Immunol. 2020;21:108322.
Nigrovic PA, Colbert RA, Holers VM, Ozen S, Ruperto N, Thompson SD, et al. Biological classification of childhood arthritis: roadmap to a molecular nomenclature. Nat Rev Rheumatol. 2021;17(5):257–69. https://doi.org/10.1038/s41584-021-00590-6.
Hinks A, Marion MC, Cobb J, Comeau ME, Sudman M, Ainsworth HC, et al. Brief report: the Genetic Profile of Rheumatoid Factor-Positive Polyarticular Juvenile Idiopathic Arthritis Resembles That of Adult Rheumatoid Arthritis. Arthritis Rheumatol. 2018;70(6):957–62.
Hinks A, Bowes J, Cobb J, Ainsworth HC, Marion MC, Comeau ME, et al. Fine-mapping the MHC locus in juvenile idiopathic arthritis (JIA) reveals genetic heterogeneity corresponding to distinct adult inflammatory arthritic diseases. Ann Rheum Dis. 2017;76(4):765–72.
Weiss PF, Fuhlbrigge RC, von Scheven E, Lovell DJ, Colbert RA, Brunner HI, et al. Children with enthesitis-related arthritis could benefit from treatments targeted for adults with spondyloarthritis. Arthritis Care Res (Hoboken). 2020.
Nigrovic PA, Raychaudhuri S, Thompson SD. Review: genetics and the classification of arthritis in adults and children. Arthritis Rheumatol. 2018;70(1):7–17.
Nigrovic PA, Martinez-Bonet M, Thompson SD. Implications of juvenile idiopathic arthritis genetic risk variants for disease pathogenesis and classification. Curr Opin Rheumatol. 2019;31(5):401–10.
Nigrovic PA, Colbert RA, Holers VM, Ozen S, Ruperto N, Thompson SD, et al. Biological classification of childhood arthritis: roadmap to a molecular nomenclature. Nat Rev Rheumatol. 2021;17(5):257–69.
Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. 2019;46(2):190–7.
Hayworth JL, Turk MA, Nevskaya T, Pope JE. The frequency of uveitis in patients with juvenile inflammatory rheumatic diseases. Joint Bone Spine. 2019;86(6):685–90.
Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res (Hoboken). 2019;71(6):703–16.
Simon S, Whiffen J, Shapiro F. Leg-length discrepancies in monoarticular and pauciarticular juvenile rheumatoid arthritis. J Bone Joint Surg Am. 1981;63(2):209–15.
Brabnikova Maresova K. Secondary osteoporosis in patients with juvenile idiopathic arthritis. J Osteoporos. 2011; p. 569417. https://www.hindawi.com/journals/jos/2011/569417/.
Min M, Hancock DG, Aromataris E, Crotti T, Boros C. Experiences of living with juvenile idiopathic arthritis: a qualitative systematic review protocol. JBI Evid Synth. 2020;18(9):2058–64.
Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Rheumatol. 2019;71(6):846–63.
Onel K. American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis (JIA): therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis and systemic JIA. Arthritis Rheumatol. 2021 (in press).
Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395–404.
Zhang X, Chen YC, Terao K. Clinical pharmacology of tocilizumab for the treatment of polyarticular-course juvenile idiopathic arthritis. Expert Rev Clin Pharmacol. 2017;10(5):471–82.
De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2385–95.
Urien S, Bardin C, Bader-Meunier B, Mouy R, Compeyrot-Lacassagne S, Foissac F, et al. Anakinra pharmacokinetics in children and adolescents with systemic-onset juvenile idiopathic arthritis and autoinflammatory syndromes. BMC Pharmacol Toxicol. 2013;5(14):40.
Falvey S, Shipman L, Ilowite N, Beukelman T. Methotrexate-induced nausea in the treatment of juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15(1):52.
Guzman J, Kerr T, Ward LM, Ma J, Oen K, Rosenberg AM, et al. Growth and weight gain in children with juvenile idiopathic arthritis: results from the ReACCh-Out cohort. Pediatr Rheumatol Online J. 2017;15(1):68.
d’Angelo DM, Di Donato G, Breda L, Chiarelli F. Growth and puberty in children with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2021;19(1):28.
Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344(6185):1249783.
Schulert GS, Yasin S, Carey B, Chalk C, Do T, Schapiro AH, et al. Systemic juvenile idiopathic arthritis-associated lung disease: characterization and risk factors. Arthritis Rheumatol. 2019;71(11):1943–54.
Saper VE, Chen G, Deutsch GH, Guillerman RP, Birgmeier J, Jagadeesh K, et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis. 2019;78(12):1722–31.
Russo RA, Katsicas MM. Patients with very early-onset systemic juvenile idiopathic arthritis exhibit more inflammatory features and a worse outcome. J Rheumatol. 2013;40(3):329–34.
Binstadt BA, Nigrovic PA. The conundrum of lung disease and drug hypersensitivity-like reactions in systemic Juvenile idiopathic arthritis. Arthritis Rheumatol 2022 (in press).
van den Brand JA, van Dijk PR, Hofstra JM, Wetzels JF. Cancer risk after cyclophosphamide treatment in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9(6):1066–73.
Choi DK, Helenowski I, Hijiya N. Secondary malignancies in pediatric cancer survivors: perspectives and review of the literature. Int J Cancer. 2014;135(8):1764–73.
Teepen JC, van Leeuwen FE, Tissing WJ, van Dulmen-den BE, van den Heuvel-Eibrink MM, van der Pal HJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER study cohort: role of chemotherapy. J Clin Oncol. 2017;35(20):2288–98.
Vanni KMM, Berliner N, Paynter NP, Glynn RJ, MacFadyen J, Colls J, et al. Adverse effects of low-dose methotrexate in a randomized double-blind placebo-controlled trial: adjudicated hematologic and skin cancer outcomes in the cardiovascular inflammation reduction trial. ACR Open Rheumatol. 2020;2(12):697–704.
United States Federal Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. 2021.
Dunne J, Rodriguez WJ, Murphy MD, Beasley BN, Burckart GJ, Filie JD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128(5):e1242–9.
Zimmerman KO, Smith PB, McMahon AW, Temeck J, Avant D, Murphy D, et al. Duration of pediatric clinical trials submitted to the us food and drug administration. JAMA Pediatr. 2019;173(1):60–7.
Ruperto N, Vesely R, Saint-Raymond A, Martini A, Paediatric Rheumatology International Trials O. Impact of the European paediatric legislation in paediatric rheumatology: past, present and future. Ann Rheum Dis. 2013;72(12):1893–6.
Ruperto N, Martini A, Pistorio A, Paediatric Rheumatology International Trials O. To randomize, or not to randomize, that is the question. Arthritis Rheumatol. 2021;73(10):1776–9.
Singh R, Ivaturi VD, Penzenstadler J, Liu T, Chen J, Marathe A, et al. Response similarity assessment between polyarticular juvenile idiopathic arthritis and adult rheumatoid arthritis for biologics. Clin Pharmacol Ther. 2021;110(1):98–107.
Brunner HI, Schanberg LE, Kimura Y, Dennos A, Co DO, Colbert RA, et al. New medications are needed for children with juvenile idiopathic arthritis. Arthritis Rheumatol. 2020;72(11):1945–51.
Beukelman T, Anink J, Berntson L, Duffy C, Ellis JA, Glerup M, et al. A survey of national and multi-national registries and cohort studies in juvenile idiopathic arthritis: challenges and opportunities. Pediatr Rheumatol Online J. 2017;15(1):31.
Shiff NJ, Beukelman T. Pharmacosurveillance in Juvenile Idiopathic Arthritis. Rheum Dis Clin N Am. 2021;47(4):643–53.
Beukelman T, Xie F, Baddley JW, Chen L, Mannion ML, Saag KG, et al. The risk of hospitalized infection following initiation of biologic agents versus methotrexate in the treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2016;18(1):210.
Beukelman T, Xie F, Chen L, Horton DB, Lewis JD, Mamtani R, et al. Risk of malignancy associated with paediatric use of tumour necrosis factor inhibitors. Ann Rheum Dis. 2018;77(7):1012–6.
Balevic SJ, Becker ML, Cohen-Wolkowiez M, Schanberg LE. Clinical trial design in juvenile idiopathic arthritis. Paediatr Drugs. 2017;19(5):379–89.
United States Federal Drug Administration. Accelerating Drug Development for Polyarticular Juvenile Idiopathic Arthritis (pJIA). 2019.
United States Federal Drug Administration. Specific requirements on content and format of labeling for human prescription drugs: revision of “pediatric use” subsection in the labeling: final rule. Fed Regist. 1994;59.
Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396–406.
United States Federal Drug Administration. NDA/BLA Multi-Disciplinary Review and Evaluation. 2019. https://www.fda.gov/media/143318/download.
Chang MH, Nigrovic PA. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight. 2019;4(5).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
PAN is funded by NIAMS awards 2R01AR065538, R01AR075906, R01AR073201, 2P30AR070253; the Fundación Bechara; and the Arbuckle Family Fund for Arthritis Research.
Conflicts of interest
PAN receives investigator-initiated research grants from Bristol-Myers Squibb and Pfizer; consulting from Bristol-Myers Squibb (BMS), Cerecor, Exo Therapeutics, Miach Orthopedics, Novartis, and Pfizer; royalties from UpToDate Inc.; and salary support from the Childhood Arthritis and Rheumatology Research Alliance.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
all materials were publicly available.
Code availability
Not applicable.
Authors' contributions
PAN and SC made substantial contributions to the conception/design of the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Both authors read and approved the final version.
Rights and permissions
About this article
Cite this article
Case, S.M., Nigrovic, P.A. Implications of Evolving Disease Classification for Drug Approval in Juvenile Idiopathic Arthritis. Pediatr Drugs 24, 185–191 (2022). https://doi.org/10.1007/s40272-022-00496-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00496-0