Abstract
In recent years, an increase in the discovery and development of biotherapeutics employing new modalities, such as bioconjugates or novel routes of delivery, has created bioanalytical challenges. The inherent complexity of conjugated molecular structures means that quantification of the bioconjugate and its multiple components is critical for preclinical/clinical studies to inform drug discovery and development. Moreover, bioconjugates involve additional multifactorial complexity because of the potential for in vivo catabolism and biotransformation, which may require thorough investigations in multiple biological matrices. Furthermore, excipients that enhance absorption are frequently evaluated and employed for the development of oral and inhaled biotherapeutics. Risk-benefit assessments are required for novel or existing excipients that utilize dosages above previously approved levels. Bioanalytical methods that can measure both excipients and potential drug metabolites in biological matrices are highly relevant to these emerging bioanalysis challenges. We discuss the bioanalytical strategies for analyzing bioconjugates such as antibody–drug conjugates and antibody–oligonucleotide conjugates and review recent advances in bioanalytical methods for the quantification and characterization of novel bioconjugates. We also discuss bioanalytical considerations for both biotherapeutics and excipients through novel administration routes and review analyses in various biological matrices, from the extensively studied serum or plasma to tissue biopsy in the context of preclinical and clinical studies from both technical and regulatory perspectives.
Similar content being viewed by others
The increasing use of new modalities such as bioconjugates and novel delivery routes in biotherapeutics brings challenges to the bioanalytical field. |
Sophisticated bioanalytical methods and comprehensive strategies are crucial for the success of drug discovery and the development of these emerging therapeutic approaches. |
Past experience with and knowledge gained from the use of small and large molecules can help guide the bioanalytical strategies and methods development for new modalities and novel delivery routes. |
1 Introduction
Biotherapeutics have been defined to date as a class of drugs that are derived from a living organism and utilized for the treatment, prevention, or cure of disease in humans, but emerging technologies may require an updated definition [1, 2]. Compared with synthetic chemical drugs, biotherapeutics often have the advantage of highly selective targeting, potentially limiting off-target interactions and thus adverse events [3]. Modern biotherapeutics emerged in the late twentieth century and quickly expanded into a variety of therapeutic areas, with diverse modalities such as peptides, cytokines, enzymes, and antibodies [4,5,6,7]. With recent advancements in protein engineering, biotherapeutics have further expanded to novel delivery routes and advanced bioconjugates to allow for highly specific targeted delivery of potent drugs, leading to improved therapeutic indexes (TI) and thus patient experiences [8, 9].
One of the primary goals of drug discovery and development is to optimize the TI by increasing the drug’s efficacy and minimizing associated toxicities. Modern drug development includes an array of different therapeutic modalities aimed at improving the TI and potentially treating hitherto underserved patient populations. Bioconjugates are chemical fusions of several molecular entities with at least one being a biomolecule. For an antibody–drug conjugate (ADC), a type of bioconjugate, an antibody targets a specific receptor expressed by tumor cells and an attached drug mediates the therapeutic response. Historically, the dominant form of bioconjugates has been ADCs aimed at the oncology setting, with increased numbers of approvals in the past few years. Recently, the implementation of bioconjugate therapeutics has been expanding to immunosuppressive, anti-inflammatory, and antimicrobial indications, among others [9].
Besides maximizing the TI, an important consideration for drug development is patient centricity. It is critical that the medication administration is convenient and comfortable. This would benefit patient compliance and thus maximize the therapeutic potential of the medication. Traditional routes of administration of biologic drugs have been largely limited to various injection routes: subcutaneous, intramuscular, or intravenous. Alternatively, microneedle technology is being used for transdermal drug delivery. It uses micro-scale needles to penetrate the stratum corneum without damaging the capillaries or nerves. However, it can cause skin irritation and allergic reactions [10,11,12]. Other drug administration methods, especially noninvasive routes (e.g., oral or inhalation), are of great interest for their patient centricity. For example, oral drugs can be easily self-administered, and inhaled drugs can achieve rapid absorption and directly target the airways to treat respiratory diseases while minimizing systemic exposure to the drug, thus increasing its TI [13]. However, significant challenges exist for biotherapeutic delivery via noninvasive routes as biologics are subject to degradation and absorption challenges [14,15,16,17]. Biotherapeutics may have limited absorption from the administration site to circulation and thus require more sensitive bioanalytical methodologies for characterization of their pharmacokinetics in circulation. Emerging research into the biodistribution of biotherapeutics further adds to the complexity of bioanalysis because of the sensitivity and selectivity challenges associated with certain tissue types. As a result, the selection of appropriate bioanalytical methodologies (e.g., enzyme-linked immunosorbent assay [ELISA], liquid chromatography-mass spectrometry [LC-MS], and polymerase chain reaction [PCR]) is critical to generate the exposure data necessary to inform drug development. Critically, the bioanalytical methodology employed should demonstrate consistent performance throughout the drug-development cycle to enable preclinical or clinical implementation, especially for studies supporting regulatory submissions. Moreover, compared with the conventional injection administration routes—where absorption and biodistribution characteristics can have lower variability—greater intersubject and interoccasion variability is common for oral or inhaled biotherapeutics, thus presenting an additional bioanalytical challenge. For example, for respiratory drugs, the particle size of the droplets and the depth of the inhaled breath can result in significant differences in the amount of drug absorbed [18]. Another challenge arises from the interest in better understanding the biotherapeutics’ biotransformations and their potential impact on the TI [19].
Bioanalytical strategy for each drug candidate must be designed to address specific questions and challenges associated with the development of a given drug. Analytical methodology, identity of analytes, and the timing of method application are critical considerations contributing to the formation of bioanalytical strategy. Bioanalytical support for novel bioconjugates and inhaled/oral biotherapeutic drugs can be very broad, including pharmacokinetic assessment, biomarker discovery, immunogenicity assessment, metabolite identification and quantification, tissue biodistribution, and co-medication quantification, among others. The scope of this article is limited to the analysis of active pharmaceutical ingredients (APIs), excipients, and their catabolites/metabolites in biological matrices. The first part of this article focuses on the discussion of bioanalysis for novel bioconjugates. We discuss general bioanalytical methods and strategies for ADC and antibody–oligonucleotide conjugate (AOC) quantification and cover applications of high-resolution mass spectrometry (HRMS) in biotransformation for bioconjugates. Method validation/qualification considerations are discussed in the context of current regulatory expectations. The second part focuses on bioanalytical considerations of biotherapeutics by novel routes of administration. Potential bioanalytical impacts from excipients on API quantification are included. Finally, bioanalytical considerations for various biological matrices, as well as ADME (absorption, distribution, metabolism, and excretion), are applicable to both novel bioconjugates and biologics delivered via novel routes. The structural complexity and potential in vivo biotransformations for bioconjugates and novel routes of administration for biotherapeutics create unique challenges for bioanalysis and call for sophisticated bioanalytical support.
2 Novel Bioconjugates
The promise of delivering therapeutic agents in a targeted fashion to increase their TI underpins the bioconjugate drug modality field. Therapeutic conjugates include diverse molecules, such as ADCs, fusion proteins, proteolysis-targeting chimeras, and others [20]. The recently approved moxetumomab pasudotox is an example of an immunotoxin that emerged conceptually decades ago as an early prototype for delivering a toxin selectively to tumor cells using a fusion protein [21, 22]. In 2021, the US FDA granted accelerated approval of melphalan flufenamide for heavily pretreated myeloma, resulting in the first peptide–drug conjugate approved for medical use [23, 24]. Although most ADCs are directed towards oncology indications, several applications have emerged outside the field of oncology [25,26,27]. Noncytotoxic payload conjugates have been drawing increased interest [28,29,30]. One recent example is ABBV-3373, which is currently being investigated for the treatment of rheumatoid arthritis [31]. It is an ADC designed to target activated immune cells instead of tumor cells. ABBV-3373 consists of a glucocorticoid payload modulating tumor necrosis factor-mediated inflammatory pathways conjugated to adalimumab [32].
New technologies that modify molecular structures to increase the TI are being applied to bioconjugate design. Probody® drug conjugate employs a masking peptide protecting the antibody complementarity determining region to limit the binding to healthy tissues. Proteases present in the tumor environment could cleave the masking peptide, which allows the drug to bind to tumor cells [33]. Bioanalytical methods for Probody® analyses should quantitatively monitor the masking peptide in the circulation [34]. One potential LC-MS method development challenge for Probody® ADCs could emerge if the surrogate analyte peptide employed to monitor the masking peptide has poor ionization efficiency or chromatographic characteristics. Although this issue is fairly common for protein/peptide bioanalysis, it can be particularly difficult in this instance as the choice is limited by the sequence of the masking peptide. Another effort involving noncytotoxic payload ADCs is using proprietary monodisperse polysarcosine (PSAR) link technology. It uses a synthetic PSAR unit that is highly hydrophilic to provide “hydrophilic shielding” for the drug payload. This approach increases the stability, homogeneity, and drug–antibody ratio (DAR) [35]. In addition, ADC molecules containing a bispecific antibody backbone or dual payloads have attracted more attention in recent years [36,37,38]. The structure of an antibody with two different arms can complicate biotransformation analyses because of the impact from potentially different conjugation sites on each antibody arm. Dual payload ADCs require bioanalytical methods that can quantify both payloads individually, which adds to the bioanalytical complexity.
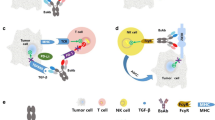
We focus on bioconjugates that consist of an antibody covalently connected with various therapeutic molecules (e.g., warhead, oligonucleotide) through a chemical linker. Bioconjugates are designed to employ a vehicle (e.g., antibody) to deliver the passenger (therapeutic molecule drug) to the destination (target cell) in a targeted manner. The heterogeneous nature of bioconjugates leads to an increased complexity of the analytes that must be measured to fully understand their pharmacokinetics, metabolism, efficacy, and safety. Bioanalytical strategies must be designed to address comprehensive characterization of the pharmacokinetic/toxicokinetic and pharmacodynamic properties of the drug candidates. Thus, methods with increasing sensitivity and specificity as well as the ability to quantify various heterogenous forms of the drug substance and biotransformations, while taking advantage of multiplexing, are highly desirable for novel biotherapeutics (Fig. 1).
To address the emerging complexity of bioconjugate bioanalysis, multiple methods may be required to support a single study. However, it is helpful to apply consistent bioanalytical strategies and methods to projects with drug candidates that share similar structural characteristics, enabling data comparability and thereby contributing to the establishment of a bioanalytical platform strategy for a given modality. The data generated for various molecules could then be compared and analyzed to guide future drug discovery and development.
2.1 Antibody–Drug Conjugates (ADCs)
Recently, ADCs have seen steady increases in regulatory approvals, with three approvals in 2019 (polatuzumab vedotin, trastuzumab deruxtecan, and enfortumab vedotin), two approvals in 2020 (sacituzumab govitecan and belantamab mafodotin), and two approvals in 2021 (loncastuximab tesirine-lpyl and tisotumab vedotin-tftv). So far, the FDA has approved 11 ADCs in the past two decades [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. Additionally, clinical trials are at various stages of evaluating the therapeutic potential of ADCs [27]. Table 1 summarizes the bioanalytical methods supporting regulatory submissions for selected FDA-approved ADC drugs.
The three fundamental assays used to assess the exposure and catabolism of ADCs are total antibody, total conjugated warhead (ADC assay), and unconjugated warhead. Depending on the structure of the ADC molecule and the characteristics of the warhead, additional assays may provide a better understanding of the pharmacokinetics and ADME of the molecule. LC-MS/MS is usually utilized for small-molecule unconjugated warhead analysis. Total antibody and ADC could be quantified using either ligand-binding assay (LBA) or LC-MS methods.
Early-stage projects typically focus on lead selection and optimization. Therefore, rapid, high-throughput, and efficient pharmacokinetic characterization is typically desired. For humanized monoclonal antibodies (mAbs) or ADCs in animal studies, generic quantification methods can be achieved using either an LBA or an LC-MS assay format. A universal LBA method for total antibody is often used for the initial pharmacokinetic evaluation, with general antihuman immunoglobulin antibodies or ligands as the capture and/or detection reagents. Applying LBA methods to quantify ADCs requires the use of a selective reagent against the specific warhead. The generation of antipayload antibody is time consuming and may be difficult during payload optimization where multiple different warhead/linker variants are being assessed simultaneously. Alternatively, for ADCs with cleavable linkers, a hybrid LBA–LC-MS/MS method measures both total antibody and conjugated warhead concentration with only one capture reagent. It utilizes enzymes to release peptides or warheads that can be separated by LC and detected by MS using a multiple reaction monitoring mode. Unlike LBA, the requirement for capture reagent selectivity/specificity is frequently less stringent for mass spectrometry-based methods, which in turn rely on surrogate detection peptides. Several fragment crystallizable (Fc) region common peptides unique to the human immunoglobulin framework are often selected as detection peptides for animal studies. The qualification of a bioanalytical method in support of discovery and early development work can follow a fit-for-purpose design, generally to minimally evaluate accuracy, precision, and selectivity for any quantification method prior to sample analysis.
For drug candidates being evaluated in good laboratory practice toxicology or clinical studies, a validated, robust, and high-throughput bioanalytical method that meets the expectations of relevant regulatory authorities is necessary to support such studies and long-term sample testing [61,62,63]. In support of clinical studies, methods employing highly selective capture antibodies are often required to achieve the necessary sensitivity and selectivity in the human matrix for ADCs employing human or humanized antibody scaffolds. For LBA, a pair of anti-idiotype antibodies are typically needed. Alternatively, LBA–LC-MS methods require only one capture antibody with a proteotypic peptide (typically from the complementarity determining region) to achieve the selectivity required. The reagent acquisition should happen sufficiently early to enable clinical assay development. Although multiple methods/approaches can frequently address bioanalytical challenges, the choice should be carefully considered for each phase of the project lifecycle and within the larger context of an overall bioanalytical strategy.
The inherent structural complexity and heterogeneity of ADCs could result in a plethora of drug catabolites/metabolites. To more comprehensively study candidate ADCs, bioanalytical methods that can address this challenge are critical for appropriate characterization of ADC pharmacokinetics. HRMS enables identification of ADC biotransformation [64,65,66].
In summary, bioanalytical methods for ADC quantification fall into two categories: LBA and hybrid LBA–LC-MS. The advantages and challenges with both platforms have been summarized previously. Briefly, LBA offers high throughput and low equipment cost and has played a critical role in the pharmacokinetic assessments of several approved ADCs [67,68,69,70]. However, when there is more interest in the structural and biotransformation information, or critical reagents are not available, LBA–LC-MS methods have the advantage and therefore have been frequently used for ADC bioanalysis.
A hybrid LBA–LC-MS/MS method detecting surrogate peptide and conjugated warhead provides unique advantages. This approach offers a DAR-sensitive bioanalytical method, generating pharmacokinetic data that can inform the deconjugation of the ADC. When the interchain disulfide bonds are disrupted because of payload conjugation employing cysteines, the heavy–light chain dissociation could affect the stability, safety, and efficacy of an ADC [71]. Information regarding antibody integrity can be obtained by measuring surrogate analyte heavy and light chain peptides. Biotransformations such as payload de-acetylation and antibody deamidation that may impact drug efficacy or safety have been reported to be quantitatively monitored by validated LC-MS methods [72, 73].
2.2 Antibody–Oligonucleotide Conjugates
Compared with ADCs, the development of AOCs as drug candidates is still in the early stages. Although several preclinical studies have been reported, AOCs are yet to enter clinical development [9, 74,75,76]. Additional bioanalytical challenges exist for AOC drug development. ADCs and AOCs share conceptually similar designs and contain three components: antibody, linker, and an active moiety (warhead or oligonucleotide chain). Learning from the experience of ADC bioanalysis, quantification of total antibody and total AOC (conjugated oligonucleotide) in the circulation of the drug candidate would be recommended. Data from total antibody and total AOC assays could provide information about oligonucleotide–antibody ratio changes in vivo. A more rapid decrease in total AOC concentrations than the total antibody concentrations has been observed in mouse studies, suggesting deconjugation of small interfering RNA (siRNA) from the antibody [77]. Because free oligonucleotides accumulate in highly perfused tissues, the detection of free oligonucleotides in circulation is challenging. Thus, it is important to assess oligonucleotide concentrations in typical accumulation sites (e.g., liver, kidney, and spleen) in preclinical studies to establish a comprehensive drug toxicokinetic profile and to inform on the overall safety profile of the AOC [78]. In addition, it would be very helpful to understand the potential efficacy and toxicity of AOCs by studying their biotransformation and catabolism using HRMS in preclinical and early clinical phases. From a regulatory perspective, full method validation should be required for total antibody and total AOC measurements in circulation. Fit-for-purpose, qualified assays would be appropriate for tissue-based analyses. Appropriate method performance evaluation should be conducted, encompassing accuracy, precision, and selectivity for AOC and free oligonucleotide measurements prior to sample analysis [79,80,81].
So far, AOC bioanalytical literature has been limited. Tan et al. [77] developed a real-time antigen capture reverse transcription PCR (RT-PCR) assay that can quantitatively detect intact antibody–siRNA conjugates in mouse serum with a lower limit of quantification (LLOQ) of 580 pg/mL. The study evaluated the potential interference from the presence of unconjugated antibody resulting from siRNA degradation in vivo [77]. Humphreys et al. [82] described a triplex-forming oligonucleotide ELISA method using locked nucleic acid containing oligonucleotide probes for quantification of antibody–siRNA conjugates in mouse serum and mouse liver homogenate. They achieved a sensitivity of 120 pg/mL, which can be further improved with proper characterization and optimization of the locked nucleic acid probe. They also demonstrated antibody–siRNA duplex and triplex conjugates using a native MS approach [82]. Both methods can achieve pg/mL level sensitivity, but their robustness still needs to be demonstrated by method validation prior to their application in studies supporting regulatory submissions.
Despite the limited information available to date on AOC bioanalytical methods, numerous ADC and oligonucleotide bioanalysis publications shed light on the direction of future method development [69, 83,84,85]. As with ADCs, total antibody can be quantified with LBA or hybrid LBA–LC-MS/MS methods. Historically, higher sensitivity can be achieved with an LBA approach, whereas the LC-MS platform can offer the flexibility of a generic capture approach [71, 86]. This is contingent upon assay requirements and available reagents. However, recent advances in mass spectrometry instrumentation have been challenging this paradigm [87]. In addition to the two aforementioned methods for total AOC quantification, native MS could be a choice but would be limited by sensitivity [82]. Denaturing intact MS methods could provide oligonucleotide–antibody ratio, biotransformation, and catabolism information with improved sensitivity over native MS. Alternatively, conjugated oligonucleotide could be released from antibody backbone using enzymatic digestion, followed by LBA, quantitative PCR, or LC-MS-based methods.
2.3 Applications of High-Resolution Mass Spectrometry for Biotransformation Analyses of ADCs
Recent progress in HRMS instrumentation and applications has enabled advanced characterization of a multitude of diverse biotherapeutics. As mentioned, ADCs, with their inherently complex structure, present significant challenges because of their numerous biotransformations. This is particularly true for ADCs that employ noncleavable linkers or non-site-specific conjugation and/or possess inhomogeneous DAR profiles. Intact HRMS methods can provide complementary information to surrogate analyte methods, thus informing drug discovery and development. Compared with the bottom-up surrogate peptide approach, intact analysis methods detect a macromolecule as a whole or components (e.g., released fragment antigen-binding region in the partial proteolysis approach). The sensitivity of intact HRMS quantification has increased significantly. Qiu et al. [88] reported an intact protein assay that could achieve an LLOQ of 50 ng/mL for mAB in mouse plasma. This level of sensitivity is close to that achieved by a typical surrogate peptide LC-MS method. This work also demonstrated that an intact analysis approach can provide equivalent quantification results (within ± 25%) when compared with a surrogate peptide method in an in vivo monkey pharmacokinetics study [88]. Zhang et al. [89] compared data generated by intact HRMS quantification and LBA assay of an mAb in a cynomolgus monkey pharmacokinetic study. Although both methods measured the entire antibody, the intact HRMS assay resulted in slightly higher concentrations than the LBA assay, thus indicating systematic bias in this case [89]. The cause of this discrepancy remains unknown but could be attributed to sample preparation procedure, instrumentation, or data processing.
In contrast to mAbs, intact mass quantification of ADCs presents additional challenges. Although Jin et al. [90] demonstrated that the quantification range with the intact HRMS method can be 5–100 μg/mL, intact quantification to ADCs faces some challenges. Heterogeneity of the drug substance itself is a significant challenge for both ADC manufacturing and bioanalysis. The ADC reference/dosing material may contain species with various DAR and additional modifications to the linker-warhead. The complex conjugation and linker–warhead structure of ADCs can present additional opportunities for in vivo biotransformation. One major biotransformation of interest is the deconjugation of the warhead over time, which, in some cases, can lead to additional catabolites, further increasing the heterogeneity. Huang et al. [91] recently presented a novel intact HRMS method applied to trastuzumab and trastuzumab-based ADCs with various DARs from a rat pharmacokinetic study. LLOQ at 1 μg/mL was achieved with 25 μL of rat plasma sample. Concentrations measured through intact and surrogate analyte approaches for the same sample were compared in this work. When applied to mAb (DAR = 0), the resulting concentration–time profiles were overlapping. However, a substantial difference was found when ADCs were analyzed, mainly originating from the biotransformations modifying the dominant species in the reference/dosing material. Although the surrogate analyte assay measured the total conjugated warhead that can be released enzymatically, the intact HRMS assay unveiled structural changes over time in dominant ADC species [91]. Thus, quantification using intact HRMS enables further assessment of previously missed individual analytes and serves as a complementary tool for a more thorough characterization of the ADC pharmacokinetics and metabolism.
3 Novel Delivery of Biologics
Besides novel bioconjugates, novel delivery routes such as inhalation or oral delivery can improve drug delivery to certain organs or benefit patient experience and thus are of increasing interest to the biopharmaceutical community. Because of its direct access to target tissues, inhalation has become an increasingly attractive route of administration of biological drugs for the treatment of respiratory diseases. Oral delivery, another noninvasive drug administration route, can significantly improve patient experience, especially in those with chronic diseases. Therefore, biopharmaceutical companies are advancing the development of biotherapeutics via nonparenteral delivery routes. For example, dornase alfa is a synthetic protein drug for patients with cystic fibrosis that aims to reduce lung infection risk and is administered via a nebulizer [92]. Cyclosporine is an oral cyclic polypeptide drug for the prevention of organ rejection [93]. Inhaled human insulin (Afrezza®) and oral semaglutide (RYBELSUS®) have been approved for the treatment of diabetes [94, 95]. Additional candidates are also in clinical development, such as AZD1402/PRS-060 and ORMD-0801 [14, 17, 96, 97].
Biotherapeutics are subject to degradation and cannot easily cross absorption barriers at physiological conditions. To address these challenges, the API properties may be modified and well-thought-out design of formulation and drug delivery devices can be applied. Excipients are ubiquitously applied to drug formulations to enhance stability, permeability, solubility, and many other properties and are crucial for the delivery of biotherapeutics via novel routes. Furthermore, sophisticated approaches such as multiunit particulate systems can also be employed to achieve controlled release of the API at given physiological conditions [98].
3.1 Excipients
Excipients are the substances in the medication other than the API or prodrug, as defined by The International Pharmaceutical Excipients Council [99]. Various excipients with a wide range of molecular mass have been utilized in drug formulations, including small molecules, macromolecules, particles (e.g., micelles, nanofibers) and even macroscopic materials (e.g., polymeric scaffolds, hydrogels). Excipients can be classified according to their various functions in formulations (Table 2) [17, 100, 101]. In addition to the excipients listed in Table 2, other types of excipients are available to improve specific drug dosage properties. For example, propyl gallate and sodium metabisulfite are commonly added to avoid oxidation. Ethylenediaminetetraacetic acid is widely used as a chelating reagent.
Excipients can also be classified as compendial and noncompendial. Compendial excipients are better characterized and are often preferred for formulation development. Information on compendial excipients used in existing FDA-approved drugs can be found in the FDA’s Inactive Ingredient Database [20]. The US Pharmacopeia-National Formulary also includes more than 5000 API and excipient standards. The FDA’s Generally Recognized as Safe notification program is another source of information about compounds that are generally considered as safe in food but that cannot be directly applied to substances that are used in new drug delivery routes at higher doses or higher frequencies [102].
On the other hand, noncompendial excipients are novel materials or materials for which the pharmacopeia monographs have not been established or have not been previously approved in a drug product. Novel noncompendial excipients are new chemical entities that are used in a medication for the first time, given via a previously unexplored route, or given at higher doses or higher frequencies than in hitherto approved drugs [25]. For example, Captisol® is a modified polyanionic beta-cyclodextrin sodium sulfonate salt employed to modulate drug solubility and stability [103, 104]. Soluplus®, a polymeric solubilizer, was introduced to the market aiming to improve the solubility and bioavailability of compounds [105]. Recombumin® is a recombinant human albumin used to stabilize therapeutic proteins and is considered a novel excipient [106].
Oral and inhaled delivery routes for biotherapeutics typically employ novel excipients in the formulation to overcome absorption challenges inherent in the novel delivery route. Following the most recent FDA guidance in Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients [107], a safety database for proposed excipients employed in new formulations needs to be established. To perform exposure–safety assessments on novel excipients and to establish exposure-based safety margins, the toxicokinetic measurements of the novel excipient and its potential metabolites in relevant biological matrices is required.
Excipients are generally considered “inactive ingredients” that are stable, and are typically nontargeting. However, whether or not certain novel excipients are truly inert compounds requires investigation before definitive conclusion. Pottel et al. [108] demonstrated that a small portion of the excipients examined may be acting on molecular targets. For example, propyl gallate, an excipient widely used in drugs, foods, and cosmetics, can inhibit 5-lipoxygenase [108]. For novel delivery, the investigation of the activity of a new excipient is critical, as excipients may perturb the pharmacology of the API. Thus, the interaction between excipients and APIs may also need careful investigation. There are multiple considerations when selecting API and excipient solutions, including charge interactions, hydrogen-donating interactions, and the reactions with lactose or silicon dioxide [109,110,111]. These reactions may potentially affect the effectiveness of APIs and the quality of the formulations. For instance, lactose can react with primary or secondary amines and facilitate the degradation of a drug through hydrolysis reaction on ester and amidine groups in vitro [112].
Even though some excipients are added to modulate API stability, they themselves may experience physical, chemical, and microbiological instability. For example, polyethylene glycol generates peroxide, which could damage proteins containing reducing functional groups [111]. Some compendial excipients are subject to changes even before dosing. Hydrolysis, oxidation, isomerization, photolysis, and polymerization are common causes of excipient degradation. For example, lactose is a widely used diluent in oral solid dosage forms. However, it is a reducing agent, which cannot coexist with strong oxidizers [100]. The impacts of excipient instability on bioanalysis are discussed in greater detail in the next section.
3.2 Bioanalytical Strategies and Considerations for Excipients and Biotherapeutics Administered via Novel Delivery Routes
Depending on the target pharmacology, adequate systemic exposure may be required for biotherapeutics delivered via a novel route of administration (Fig 2). In this case, bioanalytical assays for the quantification of biotherapeutics in circulation and at the target organ/tissue may require highly sensitive methods with a broad dynamic range that can capture low and sometimes highly variable exposures resulting from intrinsic intersubject and interoccasion variability, as well as other factors such as food effect for oral medications or history of smoking for inhaled drugs [113]. This is particularly true during the early stages of drug development, where formulations may encounter low bioavailability. Furthermore, when comparing systemic exposure from a novel delivery route with direct intravenous administration of the same compound, the potential metabolic differences need to be considered, as the compound would be exposed to different tissues, organs, and metabolic mechanisms before entering circulation [114]. Thus, additional metabolism studies may be needed for new routes of administration.
Bioanalysis of excipients is usually not conducted if well-studied components are added to the formulation. However, for novel excipients, in the context of preclinical and clinical evaluations, assessment of their pharmacokinetic profiles is necessary to properly evaluate any potential safety liability and to establish safety margins [40, 83, 115]. Introducing a novel excipient without having an independent regulatory pathway can present some risk [84].
Excipients bioanalysis largely depends on their molecular size and physicochemical properties. For instance, LC-MS/MS, as the most common tool for small-molecule bioanalysis, can be considered for excipients with lower molecular weight (e.g., small molecules, short peptides). For macromolecules, LBA approaches may be appropriate [116]. If excipients are to be evaluated in regulated studies, the bioanalytical method validation should follow the guidance set forth by the appropriate regulatory agencies [61,62,63].
In cases where excipient molecules are not inert or stable, these molecules may change because of biotransformation, oxidation, and cross reactivity. For instance, a small amount of trileucine in the formulation of an inhaled biotherapeutic can improve aerosol performance and the stability of spray-dried powder [117]. This tripeptide and its metabolite—dileucine—undergo rapid degradation in various sera matrices, suggesting that it is not logistically feasible to measure trileucine or dileucine in preclinical and clinical studies [118]. If the excipient metabolites are of potential risk, they may need to be carefully evaluated during safety studies, especially if toxicity is observed [119]. Some excipient by-products can be effectively predicted by carefully considering their structure and common biotransformation pathways. For example, a strong reducing reagent can easily become oxidized. Literature can also guide bioanalysis by providing potential metabolism information. Alongside guided predictions, untargeted analyses using HRMS can identify unexplored metabolites of excipients.
Biological drugs are frequently modified to enhance their stability and achieve optimal bioavailability. These modifications may add complexity to bioanalysis. One such approach is rational drug design. Some examples include cyclization of peptides to increase stability and the introduction of unnatural amino acids such as α,α-disubstituted amino acids to protect vulnerable proteolytic sites [120, 121]. Lipidation or use of nonproteinogenic amino acid may also contribute to the stability of the molecule [122, 123]. While increasing the stability of the compound, these modifications may affect the physicochemical properties of the compound and thus require novel bioanalytical approaches. For instance, it may be necessary to adjust the digestion conditions or to modify chromatographic methods to achieve a more suitable method. Selective antibodies may be needed to differentiate the drug product from endogenous counterparts.
Many therapeutic areas can benefit from the novel routes of administration for biotherapeutics. Nonetheless, the major focus is on chronically administered drug candidates that require self-administration. Therefore, evaluations of the pairing of biotherapeutics and excipient(s) must consider repeated dosing regimens. Additionally, analytical interference between the biotherapeutic and the excipient(s) should be assessed when co-administered. To support the bioanalysis of such drug candidates, it is good practice to demonstrate that the novel excipients at the highest expected concentration present in the biological matrix being analyzed would not affect the performance of the bioanalytical method. A common method to evaluate the potential for such an impact on recovery is to spike the excipient(s) at the highest expected concentration.
For biotherapeutics with novel delivery routes, samples in matrices other than plasma or serum are often of interest, for example tissue, feces, urine, and nasal lining fluid (NLF). Other technical bioanalytical considerations in terms of sample collection and surrogate matrix selection are discussed in detail in the following section.
4 Emerging Challenges in the Bioanalysis of Both Novel Bioconjugates and Biotherapeutics Delivered via Novel Routes
4.1 Bioanalytical Considerations for Various Biological Matrices
Relatively routine methods are established for sample processing and testing in well-characterized matrices such as plasma, serum, and urine. For biological drugs, the conventional delivery routes are subcutaneous, intramuscular, and intravenous. However, pursuit of novel administration routes and novel bioconjugates entails the integration and analysis of different biological matrices, which presents unique bioanalytical challenges.
Bioanalysis in biological matrices other than plasma, serum, or urine can offer important information on the distribution of biological drugs, which can inform mechanism of action, ADME, and/or safety considerations. For biotherapeutics with a novel route of administration, bioanalysis may be needed for the tissue(s) from corresponding absorption site(s). For ADCs, tumor distribution of ADC and released warhead can establish the exposure–efficacy relationship and may contribute to pharmacokinetic/pharmacodynamic modeling. Tissue bioanalysis is conducted mostly in preclinical studies, especially during the lead selection and lead optimization. Depending on the specific project needs, assays supporting tissue bioanalysis may vary. For most cases, a quantification assay for the drug candidate is needed. Occasionally, additional assays of major metabolites may be of more interest.
For biological drugs, the main challenge with tissue bioanalysis comes from the analyte itself. In small-molecule bioanalysis, the tissue can be disrupted in a thorough manner followed by direct precipitation or further extraction procedures using organic solvents. However, biotherapeutics typically cannot withstand such rough sample preparation procedures and maintain the capability to selectively bind to the capture reagent, which is frequently required for bioanalysis of biologic drugs. Special buffers known to retain the structural integrity of the biological drug, such as radioimmunoprecipitation assay buffer or tissue protein extraction reagent, are often used in the extraction of the analyte [124, 125]. These buffers, although gentle enough to preserve the structural integrity of the macromolecule, may result in incomplete tissue disruption and affect the extraction recovery. Therefore, in addition to tissue weight, normalization against total protein concentration may also be considered when developing methods for extracting biological analytes from tissues. On the other hand, the small-molecule format of tissue preparation can still be utilized if the analyte of interest is a small molecule, such as free warhead for bioconjugates, or a structurally modified peptide. In some cases, capture of the biotherapeutic analyte is not always necessary, as has been shown for the direct digestion approach for a cocktail of co-dosed antibodies administered at very high doses for the prevention of coronavirus disease 2019 (COVID-19) [126] and post-pellet digestion followed by solid-phase extraction (SPE) clean-up [127] of mAbs. Both of these methods have been applied to serum samples. It would be interesting to consider the application of such approaches to the bioanalysis of therapeutics from tissues that can benefit from harsher extraction conditions. Such approaches would require very careful evaluation of highly selective surrogate analyte peptides and/or extensive sample clean-up procedures.
In addition to tissue bioanalysis, NLF has been gaining more attention during the COVID-19 pandemic. Nasosorption™ FX·i is an example of a device that absorbs the biofluid from the nasal mucosa [128]. During bioanalysis, an elution solution and a device strip are added to a tube. Analytes are extracted by vortexing and centrifugation of the device stripe. This extraction process must be well characterized to establish adequate and consistent recovery [129].
Although bioconjugate analysis in unique matrices presents a clear challenge, past success in both small- and large-molecule sample preparation for pharmacokinetic/pharmacodynamic analyses can help guide bioconjugate analytical efforts. An appropriate sample preparation technique is essential to ensure the analytical performance of the method. Saliva and sputum are heterogeneous viscous matrices. This challenge has been addressed with the use of reducing agents such as dithiothreitol, which reduce protein disulfide bonds, making the matrix more homogeneous and reducing viscosity, enabling standard liquid-handling procedures [130]. Tissues such as lung or skin present difficulties because of the elastic connective tissue. Physical disruption methods, such as rotor stator homogenizers or grinding with a mortar and pestle in liquid nitrogen, are established methods that can potentially benefit bioconjugate analysis, but they are inherently limited because of throughput. Cryogenic ball mills offer a viable solution with higher throughput [131, 132]. Ultimately, it may be necessary to refine such procedures based on actual method performance.
In certain cases, bioanalysis of analytes present in biological matrices at very low quantities is required. Some examples are NLF, sputum, bronchoalveolar lavage, bone marrow aspirate, tears, and cerebrospinal fluid. When working with matrices that are difficult to obtain, a surrogate matrix approach may be warranted. Common matrices for analysis are typically readily available from commercial suppliers. However, some of the already mentioned rare matrices can be difficult or expensive to obtain. In such cases, a surrogate matrix should be employed [133]. Wakamatsu et al. [134] proposed a strategy for surrogate matrix selection for ligand-binding and LC-MS assays. For a surrogate matrix to be deemed appropriate to support quantification of a given analyte, acceptable precision, accuracy, and parallelism must be demonstrated. Matrix effect and extraction recovery evaluations in original and surrogate matrices are also required for validation. For exploratory studies where the original matrix is unavailable, full validation may be unnecessary and/or infeasible [135].
4.2 Absorption, Distribution, Metabolism, and Excretion
In contrast to small molecules, bioconjugates are structurally complex. This complexity increases for ADME studies and demands more bioanalytical methodologies necessary to support them. Mechanisms of small-molecule drug metabolism have been well established through decades of research, and the utility of this knowledge is not lost for bioconjugates, particularly for ADCs that contain a therapeutic warhead, which, as a free entity, adheres to small-molecule clearance mechanisms with similar toxicology potential [136]. However, an intact bioconjugate behaves more like a large molecule and adheres to proteolytic degradation pathways recycling the peptide structure into amino acids [137, 138]. The end result is an assortment of metabolic products ranging from small-molecule warhead metabolites to an intact bioconjugate requiring analytical support to establish therapeutic stability and a toxicology profile [139, 140]. Distribution creates analytical challenges because of the targeted nature of many bioconjugate structures. Bioconjugate structures are usually highly targeted as the antibody structure enables the therapeutic to bind to specific proteins [139]. As a consequence, bioconjugate distribution will be much higher in target than in off-target tissues. Bioanalytical strategies designed to assess tissue distribution must be able to function across multiple matrices and cover a greater range of concentrations to quantify bioconjugates across target and off-target tissues. Positron emission tomography approaches can be highly complementary to traditional bioanalytical approaches in assessing the biodistribution of bioconjugates, enabling richer temporal sampling of their distribution because of the inherently noninvasive imaging approach [141].
Bioconjugates also create unique absorption considerations. The majority of bioconjugate therapeutics on the market and in development are injected to overcome absorption challenges associated with oral or inhaled delivery routes and to mitigate expected toxicities in case of ADCs. However, as discussed, excipients themselves should be evaluated for toxicity liability, and the excipient itself may interact chemically with the bioconjugate, creating further structural complexity that bioanalytical methodology must encompass for effective quantification.
5 Future Perspectives
Bioanalysis for bioconjugates or novel routes of delivery is complex and challenging compared with traditional large- or small-molecule drugs or traditional delivery routes such as subcutaneous, intramuscular, or intravenous routes. The bioanalytical field is evolving to meet these new challenges. The importance of increasing the TI of bioconjugates such as ADCs or AOCs has driven significant improvements in optimizing the toxicity of warheads employed and/or by increasing target selectivity. As discussed, well-established bioanalytical strategies are of paramount importance for the clinical success of novel bioconjugates. With evolving technologies and instrumentation, bioanalytical methods such as LBA or hybrid LBA–LC-MS continue to serve as robust and reliable tools to better understand the pharmacokinetics, metabolism, and biodistribution of complex bioconjugates from the early stage of drug development to good laboratory practice toxicology and clinical studies. Knowledge of exposure of bioconjugates, in vivo catabolism, and biotransformation is particularly important to drive a full understanding of the efficacy and toxicity of novel bioconjugate drugs.
Patient centricity is critical for drug administration. Novel drug administration through noninvasive routes such as oral or inhaled ones are of increasing interest, and the drugs often contain novel excipients in the formulation to enhance their absorption and improve stability. Risk–benefit assessments and appropriate bioanalytical support for pharmacokinetic and safety evaluations are important when studying novel excipients. Demand is growing for highly sensitive and multiplex bioanalytical assays to address the challenges of novel route delivery, such as systemic exposure, bioavailability, or co-administered compound analytical interference.
The anticipated scholarship and improvements in drug development for novel bioconjugates or delivery routes will require innovative bioanalytical technologies to improve insights and overcome challenges in biotransformation, ADME, and tissue sample analysis, among others. Moving forward, an increased diversity of existing and novel bioanalytical methodologies will be a key factor in providing comprehensive information to help answer key questions for understanding safety and efficacy across a variety of bioconjugates and novel drug administration routes in clinical trials.
References
Cordaillat-Simmons M, Rouanet A, Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. 2020;52(9):1397–406.
Biological Product Definitions [Internet]. [cited 2021 Apr 30]. https://www.fda.gov/files/drugs/published/Biological-Product-Definitions.pdf.
Johnson DE. Biotherapeutics: challenges and opportunities for predictive toxicology of monoclonal antibodies. Int J Mol Sci. 2018;19(11):3685.
AlDeghaither D, Smaglo BG, Weiner LM. Beyond peptides and mAbs–current status and future perspectives for biotherapeutics with novel constructs. J Clin Pharmacol. 2015;55(Suppl 3):S4-20.
Zhong X, D'Antona AM. Recent advances in the molecular design and applications of multispecific biotherapeutics. Antibodies (Basel). 2021;10(2):13.
Ahmad A, Law K. Recombinant targeted proteins for biotherapy. Mol Biother. 1990;2(2):67–73.
Oldham RK. Biotherapy: the fourth modality of cancer treatment. J Cell Physiol Suppl. 1986;4:91–9.
Elgundi Z, Reslan M, Cruz E, Sifniotis V, Kayser V. The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev. 2017;122:2–19.
Leung D, Wurst JM, Liu T, Martinez RM, Datta-Mannan A, Feng Y. Antibody conjugates-recent advances and future innovations. Antibodies (Basel). 2020;9(1):2.
Dharadhar S, Majumdar A, Dhoble S, Patravale V. Microneedles for transdermal drug delivery: a systematic review. Drug Dev Ind Pharm. 2019;45(2):188–201.
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58.
Hao Y, Li W, Zhou X, Yang F, Qian Z. Microneedles-based transdermal drug delivery systems: a review. J Biomed Nanotechnol. 2017;13(12):1581–97.
Hickey AJ. Emerging trends in inhaled drug delivery. Adv Drug Deliv Rev. 2020;157:63–70.
Anselmo AC, Gokarn Y, Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discov. 2019;18(1):19–40.
Brown TD, Whitehead KA, Mitragotri S. Materials for oral delivery of proteins and peptides. Nat Rev Mater. 2019;5(2):127–48.
Morales JO, Fathe KR, Brunaugh A, Ferrati S, Li S, Montenegro-Nicolini M, et al. Challenges and future prospects for the delivery of biologics: oral mucosal, pulmonary, and transdermal routes. AAPS J. 2017;19(3):652–68.
Brown TD, Whitehead KA, Mitragotri S. Materials for oral delivery of proteins and peptides. Nat Rev Mater. 2020;5(2):127–48.
Adcock N. Bioanalysis for the development of repiratory drugs: what are the challenges. Bioanalysis. 2014;6:1143–5.
de Bono JS, Fleming MT, Wang JS, Cathomas R, Selvi Miralles M, Bothos J, et al. Phase I trial of MEDI3726, a prostate-specific membrane antigen-targeted antibody-drug conjugate, in patients with mCRPC after failure of abiraterone or enzalutamide. Clin Cancer Res. 2021;27(13):3602–9.
Rudra A, Li J, Shakur R, Bhagchandani S, Langer R. Trends in therapeutic conjugates: bench to clinic. Bioconjug Chem. 2020;31(3):462–73.
Dhillon S. Moxetumomab pasudotox: first global approval. Drugs. 2018;78(16):1763–7.
May RD, Vitetta ES, Moldenhauer G, Dorken B. Selective killing of normal and neoplastic human B cells with anti-CD19- and anti-CD22-ricin A chain immunotoxins. Cancer Drug Deliv. 1986;3(4):261–72.
FDA grants accelerated approval to melphalan flufenamide for relapsed or refractory multiple myeloma [Internet]. 2021 Mar 01 [cited 2021 Apr 30]. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-melphalan-flufenamide-relapsed-or-refractory-multiple-myeloma.
Richardson PG, Oriol A, Larocca A, Blade J, Cavo M, Rodriguez-Otero P, et al. Melflufen and dexamethasone in heavily pretreated relapsed and refractory multiple myeloma. J Clin Oncol. 2021;39(7):757–67.
Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3–19.
Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–37.
Zhao P, Zhang Y, Li W, Jeanty C, Xiang G, Dong Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm Sin B. 2020;10(9):1589–600.
Wang RE, Liu T, Wang Y, Cao Y, Du J, Luo X, et al. An immunosuppressive antibody-drug conjugate. J Am Chem Soc. 2015;137(9):3229–32.
Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527(7578):323–8.
Zhou C, Lehar S, Gutierrez J, Rosenberger CM, Ljumanovic N, Dinoso J, et al. Pharmacokinetics and pharmacodynamics of DSTA4637A: a novel THIOMAB antibody antibiotic conjugate against Staphylococcus aureus in mice. MAbs. 2016;8(8):1612–9.
A study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of ABBV-3373 in participants with moderate to severe rheumatoid arthritis [Internet]. 2021 Mar 26 [cited 2021 Apr 30]. https://ClinicalTrials.gov/show/NCT03823391.
Novel antibody drug conjugate ABBV-3373 shows improvement in disease activity in phase 2a study of patients with rheumatoid arthritis [Internet]. 2020 June 10 [cited 2021 Apr 30]. https://news.abbvie.com/news/press-releases/novel-antibody-drug-conjugate-abbv-3373-shows-improvement-in-disease-activity-in-phase-2a-study-patients-with-rheumatoid-arthritis.htm.
Autio KA, Boni V, Humphrey RW, Naing A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin Cancer Res. 2020;26(5):984–9.
Serwer L, Singh S, Krebber C, Liu S, Chauhan N, Leanna R, et al. Abstract B103: a multi-analyte HPLC-MS/MS approach to assessing exposure of a Probody drug conjugate in preclinical studies. 2018;17(1 Supplement):B103-B.
Viricel W, Fournet G, Beaumel S, Perrial E, Papot S, Dumontet C, et al. Monodisperse polysarcosine-based highly-loaded antibody-drug conjugates. Chem Sci. 2019;10(14):4048–53.
Yamaguchi A, Anami Y, Ha SYY, Roeder TJ, Xiong W, Lee J, et al. Chemical generation of small molecule-based bispecific antibody-drug conjugates for broadening the target scope. Bioorg Med Chem. 2021;32:116013.
de Goeij BE, Vink T, Ten Napel H, Breij EC, Satijn D, Wubbolts R, et al. Efficient payload delivery by a bispecific antibody-drug conjugate targeting HER2 and CD63. Mol Cancer Ther. 2016;15(11):2688–97.
Yamazaki CM, Yamaguchi A, Anami Y, Xiong W, Otani Y, Lee J, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. 2021;12(1):3528.
FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma [Internet]. 2020 Aug 06 [cited 2021 Apr 30]. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma.
FDA grants accelerated approval to sacituzumab govitecan-hziy for metastatic triple negative breast cancer [Internet]. 2020 Apr 22 [cited 2021 Apr 30]. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-sacituzumab-govitecan-hziy-metastatic-triple-negative-breast-cancer.
FDA grants accelerated approval to enfortumab vedotin-ejfv for metastatic urothelial cancer [Internet]. 2019 Dec 18 [cited 2021 Apr 30]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-type-therapy-treat-advanced-urothelial-cancer.
FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer [Internet]. 2019 Dec 23 [cited 2021 Apr 30]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-option-patients-her2-positive-breast-cancer-who-have-progressed-available.
FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma [Internet]. 2019 June 10 [cited 2021 Apr 30]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-chemoimmunotherapy-regimen-patients-relapsed-or-refractory-diffuse-large-b-cell.
Brentuximab Vedotin (marketed as Adcetris) Information [Internet]. 2015 July 08 [cited 2021 Apr 30]. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/brentuximab-vedotin-marketed-adcetris-information.
FDA approves inotuzumab ozogamicin for relapsed or refractory B-cell precursor ALL [Internet]. 2017 Aug 17 [cited 2021 Apr 30]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-inotuzumab-ozogamicin-relapsed-or-refractory-b-cell-precursor-all.
FDA approves ado-trastuzumab emtansine for early breast cancer [Internet]. 2019 May 06 [cited 2021 Apr 30]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ado-trastuzumab-emtansine-early-breast-cancer.
FDA Approves Gemtuzumab Ozogamicin for CD33-positive AML [Internet]. 2018 Mar 26 [cited 2021 Apr 30]. https://www.fda.gov/news-events/press-announcements/fda-approves-mylotarg-treatment-acute-myeloid-leukemia.
Sehn LH, Kamdar M, Herrera AF, McMillan A, Flowers C, Kim WS, et al. Randomized phase 2 trial of polatuzumab vedotin (pola) with bendamustine and rituximab (BR) in relapsed/refractory (r/r) FL and DLBCL. 2018;36(15_suppl):7507.
Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–26.
Petrylak DP, Balar AV, O'Donnell PH, McGregor BA, Heath EI, Yu EY, et al. EV-201: results of enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer previously treated with platinum and immune checkpoint inhibitors. 2019;37(18_suppl):4505.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–51.
Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–21.
Lambert J, Pautas C, Terre C, Raffoux E, Turlure P, Caillot D, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104(1):113–9.
Amiri-Kordestani L, Blumenthal GM, Xu QC, Zhang L, Tang SW, Ha L, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014;20(17):4436–41.
Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gokbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–87.
Gopal AK, Ramchandren R, O’Connor OA, Berryman RB, Advani RH, Chen R, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120(3):560–8.
Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, et al. Efficacy and safety of loncastuximab tesirine (ADCT-402) in relapsed/refractory diffuse large B-cell lymphoma. In: 62nd American society of hematology annual meeting & exposition; 2020.
FDA grants accelerated approval to loncastuximab tesirine-lpyl for large B-cell lymphoma [Internet]. 2021 Apr 23 [cited 2021 Apr 30]. https://www.fda.gov/drugs/fda-grants-accelerated-approval-loncastuximab-tesirine-lpyl-large-b-cell-lymphoma.
FDA grants accelerated approval to tisotumab vedotin-tftv for recurrent or metastatic cervical cancer. 2021 Sep 21 [cited 2021 Nov 9].
Coleman RL, Lorusso D, Gennigens C, González-Martín A, Randall L, Cibula D, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(5):609–19.
Bioanalytical Method Validation Guidance for Industry [Internet]. 2018 [cited 2021 Apr 30]. https://www.fda.gov/media/70858/download.
Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2** [Internet]. 2011 July 21 [cited 2021 Apr 30]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf.
ICH HARMONISED GUIDELINE BIOANALYTICAL METHOD VALIDATION M10 (draft version). EMA/CHMP/ICH/172948/2019 [Internet]. 2019 Mar 13 [cited 2021 Apr 30]. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-guideline-m10-bioanalytical-method-validation-step-2b_en.pdf.
Kotapati S, Passmore D, Yamazoe S, Sanku RKK, Cong Q, Poudel YB, et al. Universal affinity capture liquid chromatography-mass spectrometry assay for evaluation of biotransformation of site-specific antibody drug conjugates in preclinical studies. Anal Chem. 2020;92(2):2065–73.
Kellie JF, Pannullo KE, Li Y, Fraley K, Mayer A, Sychterz CJ, et al. Antibody subunit LC-MS analysis for pharmacokinetic and biotransformation determination from in-life studies for complex biotherapeutics. Anal Chem. 2020;92(12):8268–77.
He J, Kaur S, Xu K. High-resolution characterization of ADCs by Orbitrap LCMS. Methods Mol Biol. 2020;2078:213–9.
Kumagai K, Aida T, Tsuchiya Y, Kishino Y, Kai K, Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020;111(12):4636–45.
Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Arrojo R, Liu D, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26(5):919–31.
Mou S, Huang Y, Rosenbaum AI. ADME Considerations and bioanalytical strategies for pharmacokinetic assessments of antibody-drug conjugates. Antibodies (Basel). 2018;7(4):41.
Zhu L, Glick J, Flarakos J. Bioanalytical challenges in support of complex modalities of antibody-based therapeutics. AAPS J. 2020;22(6):130.
Huang Y, Del Nagro CJ, Balic K, Mylott WR Jr, Ismaiel OA, Ma E, et al. Multifaceted bioanalytical methods for the comprehensive pharmacokinetic and catabolic assessment of MEDI3726, an anti-prostate-specific membrane antigen pyrrolobenzodiazepine antibody-drug conjugate. Anal Chem. 2020;92(16):11135–44.
Faria M, Peay M, Lam B, Ma E, Yuan M, Waldron M, et al. Multiplex LC-MS/MS assays for clinical bioanalysis of MEDI4276, an antibody-drug conjugate of tubulysin analogue attached via cleavable linker to a biparatopic humanized antibody against HER-2. Antibodies (Basel). 2019;8(1):11.
Bults P, Bischoff R, Bakker H, Gietema JA, van de Merbel NC. LC-MS/MS-based monitoring of in vivo protein biotransformation: quantitative determination of trastuzumab and its deamidation products in human plasma. Anal Chem. 2016;88(3):1871–7.
Sugo T, Terada M, Oikawa T, Miyata K, Nishimura S, Kenjo E, et al. Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles. J Control Release. 2016;237:1–13.
Satake N, Duong C, Yoshida S, Oestergaard M, Chen C, Peralta R, et al. Novel targeted therapy for precursor B cell acute lymphoblastic leukemia: anti-CD22 antibody-MXD3 antisense oligonucleotide conjugate. Mol Med. 2016;22:632–42.
Arnold AE, Malek-Adamian E, Le PU, Meng A, Martinez-Montero S, Petrecca K, et al. Antibody-antisense oligonucleotide conjugate downregulates a key gene in glioblastoma stem cells. Mol Ther Nucleic Acids. 2018;11:518–27.
Tan M, Vernes JM, Chan J, Cuellar TL, Asundi A, Nelson C, et al. Real-time quantification of antibody-short interfering RNA conjugate in serum by antigen capture reverse transcription-polymerase chain reaction. Anal Biochem. 2012;430(2):171–8.
Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19(10):673–94.
Welink J, Xu Y, Yang E, Wilson A, Henderson N, Luo L, et al. 2018 White Paper on Recent Issues in Bioanalysis: “A global bioanalytical community perspective on last decade of incurred samples reanalysis (ISR)” (Part 1—small molecule regulated bioanalysis, small molecule biomarkers, peptides & oligonucleotide bioanalysis). Bioanalysis. 2018;10(22):1781–801.
Fandozzi C, Evans C, Wilson A, Su D, Anderson M, Clausen V, et al. 2019 White paper on recent issues in bioanalysis: chromatographic assays (part 1—innovation in small molecules and oligonucleotides & mass spectrometric method development strategies for large molecule bioanalysis). Bioanalysis. 2019;11(22):2029–48.
Neubert H, Alley SC, Lee A, Jian W, Buonarati M, Edmison A, et al. 2020 white paper on recent issues in bioanalysis: BMV of hybrid assays, acoustic MS, HRMS, data integrity, endogenous compounds, microsampling and microbiome (part 1—recommendations on industry/regulators consensus on BMV of Biotherapeutics by LCMS, advanced application in hybrid assays, regulatory challenges in mass spec, innovation in small molecules, peptides and oligos). Bioanalysis. 2021;13(4):203–38.
Humphreys SC, Thayer MB, Campuzano IDG, Netirojjanakul C, Rock BM. Quantification of siRNA-antibody conjugates in biological matrices by triplex-forming oligonucleotide ELISA. Nucleic Acid Ther. 2019;29(3):161–6.
van Dongen WD, Niessen WM. Bioanalytical LC-MS of therapeutic oligonucleotides. Bioanalysis. 2011;3(5):541–64.
Tremblay GA, Oldfield PR. Bioanalysis of siRNA and oligonucleotide therapeutics in biological fluids and tissues. Bioanalysis. 2009;1(3):595–609.
Zhu X, Huo S, Xue C, An B, Qu J. Current LC-MS-based strategies for characterization and quantification of antibody-drug conjugates. J Pharm Anal. 2020;10(3):209–20.
Tobos CI, Sheehan AJ, Duffy DC, Rissin DM. Customizable multiplex antibody array immunoassays with attomolar sensitivities. Anal Chem. 2020;92(7):5613–9.
Development of an ultra-sensitive assay for anti-sense oligonucleotide quantification - Featuring the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready, powered by SCIEX OS Software [Internet]. [cited 2022 Feb 02]. https://sciex.com/tech-notes/biopharma/development-of-an-ultra-sensitive-assay-for-anti-sense-oligonucl?sfdcname=G-7500%20Product%20Page-TN%20Development%20of%20an%20ultra-sensitive%20assay&utm_term=G-Web%20Gate%20-%20TN%20Development%20of%20an%20ultra-sensitive%20assay.
Qiu X, Kang L, Case M, Weng N, Jian W. Quantitation of intact monoclonal antibody in biological samples: comparison of different data processing strategies. Bioanalysis. 2018;10(13):1055–67.
Zhang L, Vasicek LA, Hsieh S, Zhang S, Bateman KP, Henion J. Top-down LC-MS quantitation of intact denatured and native monoclonal antibodies in biological samples. Bioanalysis. 2018;10(13):1039–54.
Jin W, Burton L, Moore I. LC-HRMS quantitation of intact antibody drug conjugate trastuzumab emtansine from rat plasma. Bioanalysis. 2018;10(11):851–62.
Huang Y, Mou S, Wang Y, Mu R, Liang M, Rosenbaum AI. Characterization of antibody-drug conjugate pharmacokinetics and in vivo biotransformation using quantitative intact LC-HRMS and surrogate analyte LC-MRM. Anal Chem. 2021;93(15):6135–44.
Hubbard RC, McElvaney NG, Birrer P, Shak S, Robinson WW, Jolley C, et al. A preliminary study of aerosolized recombinant human deoxyribonuclease I in the treatment of cystic fibrosis. N Engl J Med. 1992;326:812–5.
Noble S, Markham A. Cyclosporin. Drugs. 1995;50:924–41.
Klonoff DC. Afrezza inhaled insulin: the fastest-acting FDA-approved insulin on the market has favorable properties. J Diabetes Sci Technol. 2014;8(6):1071–3.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Bodier-Montagutelli E, Mayor A, Vecellio L, Respaud R, Heuze-Vourc’h N. Designing inhaled protein therapeutics for topical lung delivery: what are the next steps? Expert Opin Drug Deliv. 2018;15(8):729–36.
Bruns I, Fitzgerald M, Pardali K, Gardiner P, Keeling D, Axelsson L, et al. Phase 1 evaluation of the inhaled IL-4Ra antagonist, AZD1402/PRS-060, a potent and selective blocker of IL-4Ra. Eur Respir J. 2019;54(suppl 63):OA5336.
Tyagi P, Trivedi R, Pechenov S, Patel C, Revell J, Wills S, et al. Targeted oral peptide delivery using multi-unit particulates: drug and permeation enhancer layering approach. J Control Release. 2021;338:784–91.
Qualification of Excipients for Use in Pharmaceuticals [Internet]. International Pharmaceutical Excipients Council. 2008 [cited 2021 Apr 30]. https://ipecamericas.org/sites/default/files/ExcipientQualificationGuide.pdf.
Darji MA, Lalge RM, Marathe SP, Mulay TD, Fatima T, Alshammari A, et al. Excipient stability in oral solid dosage forms: a review. AAPS PharmSciTech. 2018;19(1):12–26.
Debotton N, Dahan A. Applications of polymers as pharmaceutical excipients in solid oral dosage forms. Med Res Rev. 2017;37(1):52–97.
Burdock GA, Carabin IG. Generally recognized as safe (GRAS): history and description. Toxicol Lett. 2004;150(1):3–18.
Zhao Q, Temsamani J, Agrawal S. Use of cyclodextrin and its derivatives as carriers for oligonucleotide delivery. Antisense Res Dev. 1995;5(3):185–92.
Croyle MA, Cheng X, Sandhu A, Wilson JM. Development of novel formulations that enhance adenoviral-mediated gene expression in the lung in vitro and in vivo. Mol Ther. 2001;4(1):22–8.
Shamma RN, Basha M. Soluplus®: A novel polymeric solubilizer for optimization of Carvedilol solid dispersions: Formulation design and effect of method of preparation. Powder Technol. 2013;237:406–14.
Mead D, Pearson D, Devine M. Recombinant human albumin: applications as a biopharmaceutical excipient. Innov Pharm Technol. 2007;22:42–4.
Guidance for Industry—Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients. US FDA. 2005.
Pottel J, Armstrong D, Zou L, Fekete A, Huang X-P, Hayarpi T, et al. The activities of drug inactive ingredients on biological targets. Science. 2020;369(6502):403–13.
Narasimha Murthy S, Repka MA. Excipient stability: a critical aspect in stability of pharmaceuticals. AAPS PharmSciTech. 2018;19(1):11.
Crowley P, Martini LG. Drug-excipient interactions. Pharm Technol Eur. 2001;4:7–12.
Fathima N, Mamatha T, Qureshi HK, Anitha N, Rao JV. Drug-excipient interaction and its importance in dosage form development. J Appl Pharm Sci. 2011;1:66–71.
Badawy SIF, Badawy S, Williams RC, Gilbert DL. Effect of different acids on solid-state stability of an ester prodrug of a IIb/IIIa glycoprotein receptor antagonist. Pharm Dev Technol. 1999;4(3):325–31.
Gibbons C, Blundell J, Tetens Hoff S, Dahl K, Bauer R, Baekdal T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23(2):581–8.
New R. Oral delivery of biologics via the intestine. Pharmaceutics. 2021;13(1):18.
Kozarewicz P, Loftsson T. Novel excipients—regulatory challenges and perspectives - The EU insight. Int J Pharm. 2018;546(1–2):176–9.
Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–77.
Lechuga-Ballesteros D, Charan C, Stults CL, Stevenson CL, Miller DP, Vehring R, et al. Trileucine improves aerosol performance and stability of spray-dried powders for inhalation. J Pharm Sci. 2008;97(1):287–302.
Mu R, Huang Y, Rosenbaum AI. In vitro trileucine stability evaluation in preclinical species and human sera at 37 °C using An LC-MS/MS approach. In: Proceedings of the 68th ASMS conference on mass spectrometry and allied topics; 2020 June 1–12; Online Meeting.
Osterberg RE, See NA. Toxicity of excipients—a food and drug administration perspective. Int J Toxicol. 2003;22:377–80.
Tapeinou A, Matsoukas MT, Simal C, Tselios T. Review cyclic peptides on a merry-go-round; towards drug design. Biopolymers. 2015;104(5):453–61.
Blaskovich MA. Unusual amino acids in medicinal chemistry. J Med Chem. 2016;59(24):10807–36.
Menacho-Melgar R, Decker JS, Hennigan JN, Lynch MD. A review of lipidation in the development of advanced protein and peptide therapeutics. J Control Release. 2019;295:1–12.
Pechenov S, Revell J, Will S, Naylor J, Tyagi P, Patel C, et al. Development of an orally delivered GLP-1 receptor agonist through peptide engineering and drug delivery to treat chronic disease. Sci Rep. 2021;11(1):22521.
Ngoka LC. Sample prep for proteomics of breast cancer: proteomics and gene ontology reveal dramatic differences in protein solubilization preferences of radioimmunoprecipitation assay and urea lysis buffers. Proteome Sci. 2008;6:30.
Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, et al. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem. 2009;284(51):35839–49.
Zhong X, Nayak S, Guo L, Raidas S, Zhao Y, Weiss R, et al. Liquid chromatography-multiple reaction monitoring-mass spectrometry assay for quantitative measurement of therapeutic antibody cocktail REGEN-COV concentrations in COVID-19 patient serum. Anal Chem. 2021;93(38):12889–98.
Gong C, Zheng N, Zeng J, Aubry A-F, Arnold ME. Post-pellet-digestion precipitation and solid phase extraction: a practical and efficient workflow to extract surrogate peptides for ultra-high performance liquid chromatography–tandem mass spectrometry bioanalysis of a therapeutic antibody in the low ng/mL range. J Chromatogr A. 2015;1424(11):27–36.
Thwaites RS, Jarvis HC, Singh N, Jha A, Pritchard A, Fan H, et al. Absorption of nasal and bronchial fluids: precision sampling of the human respiratory mucosa and laboratory processing of samples. J Vis Exp. 2018(131):e56413.
Loo Y-M, McTamney PM, Arends R, Abram ME, Aksyuk A, Diallo S, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in non-human primates and has an extended half-life in humans. Sci Transl Med. 2022:abl8124. https://doi.org/10.1126/scitranslmed.abl8124. (Epub 2022 Mar 9).
Nielsen H, Hvidt S, Sheils CA, Janmey PA. Elastic contributions dominate the viscoelastic properties of sputum from cystic fibrosis patients. Biophys Chem. 2004;112(2–3):193–200.
Prieto DA, Blonder J. Chapter 3—tissue sample preparation for proteomic analysis. In: Issaq HJ, Veenstra TD, editors. Proteomic and metabolomic approaches to biomarker discovery. 2nd ed. Boston: Academic Press; 2020. p. 39–52.
Hopfgartner G, Wenkui L, Wenying J, Yunlin F. Sample preparation in LC-MS bioanalysis. Anal Bioanal Chem. 2020;412(4):803–4.
Ho S, Gao H. Surrogate matrix: opportunities and challenges for tissue sample analysis. Bioanalysis. 2015;7:2419–33.
Wakamatsu A, Ochiai S, Suzuki E, Yokota Y, Ochiai M, Kotani Y, Sasahara S, Nakanaga K, Hashimoto Y, Ueno S, Kato N. Proposed selection strategy of surrogate matrix to quantify endogenous substances by Japan Bioanalysis Forum DG2015-15. Bioanalysis. 2018;10:1349–60.
Lin K, Cabral P, Ekpenyong O, Bader SE, Galvao J, Kim Y, et al. A surrogate matrix-based approach toward multiplexed quantitation of an sGC stimulator and cGMP in ocular tissue and plasma. Toxicol Pathol. 2021;49(3):544–54.
Hochman JH. Adapting ADME and pharmacokinetic analysis to the next generation of therapeutic modalities. J Pharm Sci. 2021;110(1):35–41.
Kamath AV, Iyer S. Preclinical pharmacokinetic considerations for the development of antibody drug conjugates. Pharm Res. 2015;32(11):3470–9.
Kamath AV, Iyer S. Challenges and advances in the assessment of the disposition of antibody-drug conjugates. Biopharm Drug Dispos. 2016;37(2):66–74.
Kraynov E, Kamath AV, Walles M, Tarcsa E, Deslandes A, Iyer RA, et al. Current approaches for absorption, distribution, metabolism, and excretion characterization of antibody-drug conjugates: an industry white paper. Drug Metab Dispos. 2016;44(5):617–23.
Han TH, Zhao B. Absorption, distribution, metabolism, and excretion considerations for the development of antibody-drug conjugates. Drug Metab Dispos. 2014;42(11):1914–20.
Cahuzac H, Devel L. Analytical methods for the detection and quantification of ADCs in biological matrices. Pharmaceuticals (Basel). 2020;13(12):462.
BLA Multi-disciplinary Review and Evaluation {Biologics License Application (BLA) 761208}. 2021 Mar [cited 2021 Nov 9].
BLA 761196 Multi‐disciplinary Review and Evaluation loncastuximab tesirine. 2020 Jan [cited 2021 Nov 9].
NDA/BLA Multi-disciplinary Review and Evaluation {BLA 761139}. 2019 Jun 11 [cited 2021 Nov 9].
NDA/BLA Multi-disciplinary Review and Evaluation BLA 761115. 2018 Apr 2 [cited 2021 Nov 9].
BLA Multi-disciplinary Review and Evaluation BLA761158. 2019 Jun 11 [cited 2021 Nov 9].
BLA 761121. 2018 [cited 2021 Nov 9].
NDA/BLA Multi-disciplinary Review and Evaluation – BLA 761137. 2019 Jun 11 [cited 2021 Nov 9].
NDA/BLA Multi-disciplinary Review and Evaluation {BLA 761040}. 2016 Feb 1 [cited 2021 Nov 9].
BLA 125427. 2012 [cited 2021 Nov 9].
Acknowledgments
The authors thank Kevin Contrepois, Hui Yin Tan, and Liu Yang for providing critical feedback and Joshua Chrisafis and Hailey Kessler for helping with graphic design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Author contributions
All authors contributed to the manuscript preparation. Ruipeng Mu and Jiaqi Yuan contributed equally to this work. Anton I. Rosenbaum, Ruipeng Mu, and Jiaqi Yuan contributed to the idea of the article. Ruipeng Mu, Jiaqi Yuan, Yue Huang, John K. Meissen, and Si Mou contributed to the drafting and critical revision of this work. Meina Liang and Anton I. Rosenbaum contributed to the critical revision of this work. All authors read and approved the final manuscript.
Conflict of interest
Ruipeng Mu, Jiaqi Yuan, Yue Huang, John K. Meissen, Si Mou, Meina Liang, and Anton I. Rosenbaum are employees and shareholders of AstraZeneca.
Ethics approval
Not applicable.
Consent to participate/publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mu, R., Yuan, J., Huang, Y. et al. Bioanalytical Methods and Strategic Perspectives Addressing the Rising Complexity of Novel Bioconjugates and Delivery Routes for Biotherapeutics. BioDrugs 36, 181–196 (2022). https://doi.org/10.1007/s40259-022-00518-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00518-w