Abstract
The brain is a highly energy-demanding organ and requires bioenergetic adaptability to balance normal activity with pathophysiological fuelling of spontaneous recurrent seizures, the hallmark feature of the epilepsies. Recurrent or prolonged seizures have long been known to permanently alter neuronal circuitry and to cause excitotoxic injury and aberrant inflammation. Furthermore, pathological changes in bioenergetics and metabolism are considered downstream consequences of epileptic seizures that begin at the synaptic level. However, as we highlight in this Review, evidence is also emerging that primary derangements in cellular or mitochondrial metabolism can result in seizure genesis and lead to spontaneous recurrent seizures. Basic and translational research indicates that the relationships between brain metabolism and epileptic seizures are complex and bidirectional, producing a vicious cycle that compounds the deleterious consequences of seizures. Metabolism-based treatments such as the high-fat, antiseizure ketogenic diet have become mainstream, and metabolic substrates and enzymes have become attractive molecular targets for seizure prevention and recovery. Moreover, given that metabolism is crucial for epigenetic as well as inflammatory changes, the idea that epileptogenesis can be both negatively and positively influenced by metabolic changes is rapidly gaining ground. Here, we review evidence that supports both pathophysiological and therapeutic roles for brain metabolism in epilepsy.
Key points
-
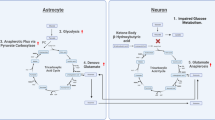
Epileptic seizures induce widespread derangements in cellular and mitochondrial metabolism, as well as cerebral flood flow.
-
Primary defects in genes that encode mitochondrial proteins and/or metabolic substrates and enzymes can increase neuronal and glial network excitability.
-
Brain metabolic homeostasis and function can be viewed as an interplay among the cerebral circulation, glia and neurons, also known as the neurovascular unit.
-
Epilepsy can be viewed as a metabolic disease, and primordial mechanisms evoked by compounds such as adenosine could be highly relevant to seizures and epileptogenesis.
-
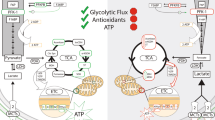
Metabolism-based treatments such as the high-fat ketogenic diet and its variants can help to restore metabolic homeostasis and enable seizure control.
-
As dietary therapies can often control seizures in individuals with medically intractable epilepsy, experimental therapeutics based on metabolic targets should be explored.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Devinsky, O. et al. Epilepsy. Nat. Rev. Dis. Prim. 4, 18024 (2018).
Hall, C. N., Klein-Flugge, M. C., Howarth, C. & Attwell, D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J. Neurosci. 32, 8940–8951 (2012).
Rangaraju, V., Calloway, N. & Ryan, T. A. Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 (2014).
Otahal, J., Folbergrova, J., Kovacs, R., Kunz, W. S. & Maggio, N. Epileptic focus and alteration of metabolism. Int. Rev. Neurobiol. 114, 209–243 (2014).
Zsurka, G. & Kunz, W. S. Mitochondrial dysfunction and seizures: the neuronal energy crisis. Lancet Neurol. 14, 956–966 (2015).
Vezzani, A., French, J., Bartfai, T. & Baram, T. Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 7, 31–40 (2011).
Vezzani, A., Pascente, R. & Ravizza, T. Biomarkers of epileptogenesis: the focus on glia and cognitive dysfunctions. Neurochem. Res. 42, 2089–2098 (2017).
Eyo, U. B., Murugan, M. & Wu, L. J. Microglia-neuron communication in epilepsy. Glia 65, 5–18 (2017).
Lim, A. & Thomas, R. H. The mitochondrial epilepsies. Eur. J. Paediatr. Neurol. 24, 47–52 (2020).
Pearson-Smith, J. N. & Patel, M. Metabolic dysfunction and oxidative stress in epilepsy. Int. J. Mol. Sci. 18, 2365 (2017).
Conboy, K., Henshall, D. C. & Brennan, G. P. Epigenetic principles underlying epileptogenesis and epilepsy syndromes. Neurobiol. Dis. 148, 105179 (2021).
Pan, J. W. et al. Neurometabolism in human epilepsy. Epilepsia 49(Suppl. 3), 31–41 (2008).
Singh, S. et al. Reviving mitochondrial bioenergetics: a relevant approach in epilepsy. Mitochondrion 58, 213–226 (2021).
Rogawski, M. A., Loscher, W. & Rho, J. M. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb. Perspect. Med. 6, a022780 (2016).
Chen, Z., Brodie, M. J., Liew, D. & Kwan, P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 75, 279–286 (2018).
Rho, J. M. & White, H. S. Brief history of anti-seizure drug development. Epilepsia Open 3, 114–119 (2018).
Lynch, B. A. et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl Acad. Sci. USA 101, 9861–9866 (2004).
Bialer, M. et al. Progress report on new antiepileptic drugs: a summary of the Fifteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XV). II. Drugs in more advanced clinical development. Epilepsia 61, 2365–2385 (2020).
Freeman, J., Veggiotti, P., Lanzi, G., Tagliabue, A. & Perucca, E. The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res. 68, 145–180 (2006).
Neal, E. G. et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 7, 500–506 (2008).
Neal, E. G. et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 50, 1109–1117 (2009).
Huttenlocher, P. R. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 10, 536–540 (1976).
Kossoff, E. et al. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia 47, 421–424 (2006).
Muzykewicz, D. A. et al. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia 50, 1118–1126 (2009).
Martin-McGill, K. J., Bresnahan, R., Levy, R. G. & Cooper, P. N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 6, CD001903 (2020).
Gano, L. B., Patel, M. & Rho, J. M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 55, 2211–2228 (2014).
Sokoloff, L. et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the concscious and anesthetized albino rat. J. Neurochem. 28, 897–916 (1977).
Belanger, M., Allaman, I. & Magistretti, P. J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 14, 724–738 (2011).
Engl, E. & Attwell, D. Non-signalling energy use in the brain. J. Physiol. 593, 3417–3429 (2015).
Profaci, C. P., Munji, R. N., Pulido, R. S. & Daneman, R. The blood-brain barrier in health and disease: important unanswered questions. J. Exp. Med. 217, e20190062 (2020).
Loscher, W. & Friedman, A. Structural, molecular, and functional alterations of the blood–brain barrier during epileptogenesis and epilepsy: a cause, consequence, or both? Int. J. Mol. Sci. 21, 591 (2020).
Dienel, G . A. & Carlson, G. M. Major advances in brain glycogen research: understanding of the roles of glycogen have evolved from emergency fuel reserve to dynamic, regulated participant in diverse brain functions. Adv. Neurobiol. 23, 1–16 (2019).
Dienel, G. A. Fueling and imaging brain activation. ASN Neuro 4, e00093 (2012).
Owen, O. E. et al. Brain metabolism during fasting. J. Clin. Invest. 46, 1589–1595 (1967).
Hasselbalch, S. G. et al. Brain metabolism during short-term starvation in humans. J. Cereb. Blood Flow. Metab. 14, 125–131 (1994).
Bordone, M. P. et al. The energetic brain–a review from students to students. J. Neurochem. 151, 139–165 (2019).
Boison, D. & Steinhauser, C. Epilepsy and astrocyte energy metabolism. Glia 66, 1235–1243 (2018).
Maher, F., Vannucci, S. J. & Simpson, I. A. Glucose transporter proteins in brain. FASEB J. 8, 1003–1011 (1994).
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994).
Magistretti, P. J. & Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018).
Machler, P. et al. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 23, 94–102 (2016).
Felmlee, M. A., Jones, R. S., Rodriguez-Cruz, V., Follman, K. E. & Morris, M. E. Monocarboxylate transporters (SLC16): function, regulation, and role in health and disease. Pharmacol. Rev. 72, 466–485 (2020).
Bak, L. K. et al. Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J. Neurochem. 109 (Suppl. 1), 87–93 (2009).
Diaz-Garcia, C. M. & Yellen, G. Neurons rely on glucose rather than astrocytic lactate during stimulation. J. Neurosci. Res. 97, 883–889 (2019).
Lin, A. L., Fox, P. T., Hardies, J., Duong, T. Q. & Gao, J. H. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc. Natl Acad. Sci. USA 107, 8446–8451 (2010).
Tarczyluk, M. A. et al. Functional astrocyte-neuron lactate shuttle in a human stem cell-derived neuronal network. J. Cereb. Blood Flow. Metab. 33, 1386–1393 (2013).
Devinsky, O., Vezzani, A., Najjar, S., De Lanerolle, N. C. & Rogawski, M. A. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 36, 174–184 (2013).
Robel, S. & Sontheimer, H. Glia as drivers of abnormal neuronal activity. Nat. Neurosci. 19, 28–33 (2016).
Verhoog, Q. P., Holtman, L., Aronica, E. & van Vliet, E. A. Astrocytes as guardians of neuronal excitability: mechanisms underlying epileptogenesis. Front. Neurol. 11, 591690 (2020).
Huguenard, J. R. & McCormick, D. A. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 30, 350–356 (2007).
Lado, F. A. & Moshe, S. L. How do seizures stop? Epilepsia 49, 1651–1664 (2008).
Yekhlef, L., Breschi, G. L., Lagostena, L., Russo, G. & Taverna, S. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J. Neurophysiol. 113, 1616–1630 (2015).
Toyoda, I., Fujita, S., Thamattoor, A. K. & Buckmaster, P. S. Unit activity of hippocampal interneurons before spontaneous seizures in an animal model of temporal lobe epilepsy. J. Neurosci. 35, 6600–6618 (2015).
Righes Marafiga, J., Vendramin Pasquetti, M. & Calcagnotto, M. E. GABAergic interneurons in epilepsy: more than a simple change in inhibition. Epilepsy Behav. 121, 106935 (2020).
Dudek, F. E., Yasumura, T. & Rash, J. E. Non-synaptic mechanisms in seizures and epileptogenesis. Cell Biol. Int. 22, 793–805 (1998).
Timofeev, I., Bazhenov, M., Seigneur, J. & Sejnowski, T. Neocortical synchronization. Epilepsia 51, 18 (2010).
Jefferys, J. G. et al. Mechanisms of physiological and epileptic HFO generation. Prog. Neurobiol. 98, 250–264 (2012).
Shivacharan, R. S. et al. Neural recruitment by ephaptic coupling in epilepsy. Epilepsia 62, 1505–1517 (2021).
Noebels, J. Pathway-driven discovery of epilepsy genes. Nat. Neurosci. 18, 344–350 (2015).
Blumcke, I. et al. Toward a refined genotype-phenotype classification scheme for the international consensus classification of focal cortical dysplasia. Brain Pathol. 31, e12956 (2021).
Barkovich, A. J., Dobyns, W. B. & Guerrini, R. Malformations of cortical development and epilepsy. Cold Spring Harb. Perspect. Med. 5, a022392 (2015).
Becker, A. J. Review: animal models of acquired epilepsy: insights into mechanisms of human epileptogenesis. Neuropathol. Appl. Neurobiol. 44, 112–129 (2018).
Crino, P. B. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat. Rev. Neurol. 12, 379–392 (2016).
Pitkanen, A., Lukasiuk, K., Dudek, F. E. & Staley, K. J. Epileptogenesis. Cold Spring Harb. Perspect. Med. 5, a022822 (2015).
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–1741.e13 (2018).
Loeb, J. A. Identifying targets for preventing epilepsy using systems biology. Neurosci. Lett. 497, 205–212 (2011).
Kirchner, A., Dachet, F. & Loeb, J. A. Identifying targets for preventing epilepsy using systems biology of the human brain. Neuropharmacology 168, 107757 (2020).
McKee, H. R. & Privitera, M. D. Stress as a seizure precipitant: identification, associated factors, and treatment options. Seizure 44, 21–26 (2017).
Walsh, S. et al. A systematic review of the risks factors associated with the onset and natural progression of epilepsy. Neurotoxicology 61, 64–77 (2017).
Chen, T., Giri, M., Xia, Z., Subedi, Y. N. & Li, Y. Genetic and epigenetic mechanisms of epilepsy: a review. Neuropsychiatr. Dis. Treat. 13, 1841–1859 (2017).
Qaiser, F., Yuen, R. K. C. & Andrade, D. M. Genetics of epileptic networks: from focal to generalized genetic epilepsies. Curr. Neurol. Neurosci. Rep. 20, 46 (2020).
Kobow, K. et al. Finding a better drug for epilepsy: antiepileptogenesis targets. Epilepsia 53, 1868–1876 (2012).
Engel, J. Jr & Pitkanen, A. Biomarkers for epileptogenesis and its treatment. Neuropharmacology 167, 107735 (2020).
Duncan, R. Epilepsy, cerebral blood flow, and cerebral metabolic rate. Cerebrovasc. Brain Metab. Rev. 4, 105–121 (1992).
Sidhu, M. K., Duncan, J. S. & Sander, J. W. Neuroimaging in epilepsy. Curr. Opin. Neurol. 31, 371–378 (2018).
Goodman, A. M. & Szaflarski, J. P. Recent advances in neuroimaging of epilepsy. Neurotherapeutics 18, 811–826 (2021).
Chugani, H. T. Hypermetabolism on pediatric positron emission tomography scans of brain glucose metabolism: what does it signify? J. Nucl. Med. 62, 1301–1306 (2021).
Alkonyi, B., Chugani, H. T. & Juhasz, C. Transient focal cortical increase of interictal glucose metabolism in Sturge-Weber syndrome: implications for epileptogenesis. Epilepsia 52, 1265–1272 (2011).
Juhasz, C., Hu, J., Xuan, Y. & Chugani, H. T. Imaging increased glutamate in children with Sturge-Weber syndrome: association with epilepsy severity. Epilepsy Res. 122, 66–72 (2016).
Nelissen, N. et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage 32, 684–695 (2006).
Rakhade, S. N. & Jensen, F. E. Epileptogenesis in the immature brain: emerging mechanisms. Nat. Rev. Neurol. 5, 380–391 (2009).
Ingvar, M. & Siesjo, B. K. Local blood flow and glucose consumption in the rat brain during sustained bicuculline-induced seizures. Acta Neurol. Scand. 68, 129–144 (1983).
Theodore, W. H. Cerebral blood flow and glucose metabolism in human epilepsy. Adv. Neurol. 79, 873–881 (1999).
Shultz, S. R., O’Brien, T. J., Stefanidou, M. & Kuzniecky, R. I. Neuroimaging the epileptogenic process. Neurotherapeutics 11, 347–357 (2014).
During, M. J., Fried, I., Leone, P., Katz, A. & Spencer, D. D. Direct measurement of extracellular lactate in the human hippocampus during spontaneous seizures. J. Neurochem. 62, 2356–2361 (1994).
Boison, D. & Yegutkin, G. G. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 36, 582–596 (2019).
Dienel, G. A. Brain glucose metabolism: integration of energetics with function. Physiol. Rev. 99, 949–1045 (2019).
Sada, N., Lee, S., Katsu, T., Otsuki, T. & Inoue, T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347, 1362–1367 (2015).
McDonald, T. S. & Borges, K. Impaired hippocampal glucose metabolism during and after flurothyl-induced seizures in mice: reduced phosphorylation coincides with reduced activity of pyruvate dehydrogenase. Epilepsia 58, 1172–1180 (2017).
Bhandary, S. & Aguan, K. Pyruvate dehydrogenase complex deficiency and its relationship with epilepsy frequency–an overview. Epilepsy Res. 116, 40–52 (2015).
McDonald, T. S., Carrasco-Pozo, C., Hodson, M. P. & Borges, K. Alterations in cytosolic and mitochondrial [U-13C]glucose metabolism in a chronic epilepsy mouse model. eNeuro 4(1), ENEURO.0341-16.2017 (2017).
Liang, L. P., Ho, Y. S. & Patel, M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience 101, 563–570 (2000).
Bainbridge, M. N. et al. Analyses of SLC13A5-epilepsy patients reveal perturbations of TCA cycle. Mol. Genet. Metab. 121, 314–319 (2017).
Smeland, O. B., Hadera, M. G., McDonald, T. S., Sonnewald, U. & Borges, K. Brain mitochondrial metabolic dysfunction and glutamate level reduction in the pilocarpine model of temporal lobe epilepsy in mice. J. Cereb. Blood Flow. Metab. 33, 1090–1097 (2013).
Kunz, W. S., Goussakov, I. V., Beck, H. & Elger, C. E. Altered mitochondrial oxidative phosphorylation in hippocampal slices of kainate-treated rats. Brain Res. 826, 236–242 (1999).
Folbergrova, J., Jesina, P., Haugvicova, R., Lisy, V. & Houstek, J. Sustained deficiency of mitochondrial complex I activity during long periods of survival after seizures induced in immature rats by homocysteic acid. Neurochem. Int. 56, 394–403 (2010).
Ryan, K., Backos, D. S., Reigan, P. & Patel, M. Post-translational oxidative modification and inactivation of mitochondrial complex I in epileptogenesis. J. Neurosci. 32, 11250–11258 (2012).
Rowley, S. & Patel, M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free. Radic. Biol. Med. 62, 121–131 (2013).
Rahman, S. Mitochondrial disease and epilepsy. Dev. Med. Child. Neurol. 54, 397–406 (2012).
Williams, S., Hamil, N., Abramov, A. Y., Walker, M. C. & Kovac, S. Status epilepticus results in persistent overproduction of reactive oxygen species, inhibition of which is neuroprotective. Neuroscience 303, 160–165 (2015).
Patel, M., Li, Q. Y., Chang, L. Y., Crapo, J. & Liang, L. P. Activation of NADPH oxidase and extracellular superoxide production in seizure-induced hippocampal damage. J. Neurochem. 92, 123–131 (2005).
Patel, M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free. Radic. Biol. Med. 37, 1951–1962 (2004).
Jarrett, S. G., Liang, L. P., Hellier, J. L., Staley, K. J. & Patel, M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol. Dis. 30, 130–138 (2008).
Patsoukis, N. et al. Thiol redox state and lipid and protein oxidation in the mouse striatum after pentylenetetrazol-induced epileptic seizure. Epilepsia 46, 1205–1211 (2005).
Mueller, S. G., Trabesinger, A. H., Boesiger, P. & Wieser, H. G. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology 57, 1422–1427 (2001).
Liang, L. P. & Patel, M. Seizure-induced changes in mitochondrial redox status. Free Radic. Biol. Med. 40, 316–322 (2006).
Beltran Gonzalez, A. N., Lopez Pazos, M. I. & Calvo, D. J. Reactive oxygen species in the regulation of the GABA mediated inhibitory neurotransmission. Neuroscience 439, 137–145 (2020).
Trevelyan, A. J., Sussillo, D. & Yuste, R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J. Neurosci. 27, 3383–3387 (2007).
Pallafacchina, G., Zanin, S. & Rizzuto, R. Recent advances in the molecular mechanism of mitochondrial calcium uptake. F1000Res 7, F1000 Faculty Rev-1858 (2018).
Giorgi, C. et al. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion 12, 77–85 (2012).
Pathak, T. & Trebak, M. Mitochondrial Ca(2+) signaling. Pharmacol. Ther. 192, 112–123 (2018).
Newby, A. C. Adenosine and the concept of ‘retaliatory metabolites’. Trends Biochem. Sci. 9, 42–44 (1984).
During, M. J. & Spencer, D. D. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol. 32, 618–624 (1992).
Dragunow, M., Goddard, G. V. & Laverty, R. Is adenosine an endogenous anticonvulsant? Epilepsia 26, 480–487 (1985).
Boison, D. et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc. Natl Acad. Sci. USA 99, 6985–6990 (2002).
Williams-Karnesky, R. L. et al. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J. Clin. Invest. 123, 3552–3563 (2013).
Mohler, H. & Okada, T. Benzodiazepine receptor: demonstration in the central nervous system. Science 198, 849–851 (1977).
Klein, P. et al. Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia 59, 37–66 (2018).
Fredholm, B. B., Chen, J. F., Cunha, R. A., Svenningsson, P. & Vaugeois, J. M. Adenosine and brain function. Int. Rev. Neurobiol. 63, 191–270 (2005).
Fredholm, B. B., Chen, J. F., Masino, S. A. & Vaugeois, J. M. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 45, 385–412 (2005).
Fredholm, B. B., Ijzerman, A. P., Jacobson, K. A., Linden, J. & Muller, C. E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol. Rev. 63, 1–34 (2011).
Boison, D. Adenosine kinase: exploitation for therapeutic gain. Pharmacol. Rev. 65, 906–943 (2013).
Kluger, G. et al. Pyridoxine-dependent epilepsy: normal outcome in a patient with late diagnosis after prolonged status epilepticus causing cortical blindness. Neuropediatrics 39, 276–279 (2008).
Malaspina, P. et al. Succinic semialdehyde dehydrogenase deficiency (SSADHD): pathophysiological complexity and multifactorial trait associations in a rare monogenic disorder of GABA metabolism. Neurochem. Int. 99, 72–84 (2016).
Tremino, L., Forcada-Nadal, A. & Rubio, V. Insight into vitamin B6-dependent epilepsy due to PLPBP (previously PROSC) missense mutations. Hum. Mutat. 39, 1002–1013 (2018).
Mills, P. B. et al. Epilepsy due to PNPO mutations: genotype, environment and treatment affect presentation and outcome. Brain 137, 1350–1360 (2014).
Mills, P. B. et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5′-phosphate oxidase. Hum. Mol. Genet. 14, 1077–1086 (2005).
Pope, S., Artuch, R., Heales, S. & Rahman, S. Cerebral folate deficiency: analytical tests and differential diagnosis. J. Inherit. Metab. Dis. 42, 655–672 (2019).
Alonso-Aperte, E., Ubeda, N., Achon, M., Perez-Miguelsanz, J. & Varela-Moreiras, G. Impaired methionine synthesis and hypomethylation in rats exposed to valproate during gestation. Neurology 52, 750–756 (1999).
Applegarth, D. A. & Toone, J. R. Glycine encephalopathy (nonketotic hyperglycinaemia): review and update. J. Inherit. Metab. Dis. 27, 417–422 (2004).
Labrie, V. & Roder, J. C. The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci. Biobehav. Rev. 34, 351–372 (2010).
Bergeron, R., Meyer, T. M., Coyle, J. T. & Greene, R. W. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl Acad. Sci. USA 95, 15730–15734 (1998).
Gramer, G. et al. Glucose transporter-1 (GLUT1) deficiency syndrome: diagnosis and treatment in late childhood. Neuropediatrics 43, 168–171 (2012).
Gloyn, A. L. et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 350, 1838–1849 (2004).
Shimomura, K. et al. A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology 69, 1342–1349 (2007).
Olson, T. M. & Terzic, A. Human KATP channelopathies: diseases of metabolic homeostasis. Pflug. Arch. 460, 295–306 (2010).
Braissant, O., McLin, V. A. & Cudalbu, C. Ammonia toxicity to the brain. J. Inherit. Metab. Dis. 36, 595–612 (2013).
Llansola, M. et al. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab. Brain Dis. 22, 321–335 (2007).
Lax, N. Z., Gorman, G. S. & Turnbull, D. M. Review: central nervous system involvement in mitochondrial disease. Neuropathol. Appl. Neurobiol. 43, 102–118 (2017).
Bindoff, L. A. & Engelsen, B. A. Mitochondrial diseases and epilepsy. Epilepsia 53 (Suppl. 4), 92–97 (2012).
Chan, F. et al. The role of astrocytes in seizure generation: insights from a novel in vitro seizure model based on mitochondrial dysfunction. Brain 142, 391–411 (2019).
Lax, N. Z. et al. Extensive respiratory chain defects in inhibitory interneurones in patients with mitochondrial disease. Neuropathol. Appl. Neurobiol. 42, 180–193 (2016).
Kossoff, E. H. et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 3, 175–192 (2018).
Pfeifer, H. H. & Thiele, E. A. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 65, 1810–1812 (2005).
Kim, S. H. et al. The ketogenic diet in children 3 years of age or younger: a 10-year single-center experience. Sci. Rep. 9, 8736 (2019).
Lyons, L., Schoeler, N. E., Langan, D. & Cross, J. H. Use of ketogenic diet therapy in infants with epilepsy: a systematic review and meta-analysis. Epilepsia 61, 1261–1281 (2020).
Husari, K. S. & Cervenka, M. C. The ketogenic diet all grown up – ketogenic diet therapies for adults. Epilepsy Res. 162, 106319 (2020).
Sondhi, V. & Gulati, S. Efficacy of 3 major ketogenic diet therapies in children with drug-resistant epilepsy–reply. JAMA Pediatr. 175, 434–435 (2021).
Sourbron, J. et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Childs Nerv. Syst. 36, 1099–1109 (2020).
Boison, D. New insights into the mechanisms of the ketogenic diet. Curr. Opin. Neurol. 30, 187–192 (2017).
Poff, A. M., Rho, J. M. & D’Agostino, D. P. Ketone administration for seizure disorders: history and rationale for ketone esters and metabolic alternatives. Front. Neurosci. 13, 1041 (2019).
McDonald, T., Puchowicz, M. & Borges, K. Impairments in oxidative glucose metabolism in epilepsy and metabolic treatments thereof. Front. Cell Neurosci. 12, 274 (2018).
DeVivo, D. C., Leckie, M. P., Ferrendelli, J. S. & McDougal, D. B. Jr Chronic ketosis and cerebral metabolism. Ann. Neurol. 3, 331–337 (1978).
Bough, K. J. et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 60, 223–235 (2006).
Kim, D. Y., Vallejo, J. & Rho, J. M. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J. Neurochem. 114, 130–141 (2010).
Milder, J. B., Liang, L. P. & Patel, M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 40, 238–244 (2010).
Juge, N. et al. Metabolic control of vesicular glutamate transport and release. Neuron 68, 99–112 (2010).
Kawamura, M. Jr., Ruskin, D. N. & Masino, S. A. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J. Neurosci. 30, 3886–3895 (2010).
Ma, W., Berg, J. & Yellen, G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J. Neurosci. 27, 3618–3625 (2007).
Shao, L. R., Rho, J. M. & Stafstrom, C. E. Glycolytic inhibition: a novel approach toward controlling neuronal excitability and seizures. Epilepsia Open 3, 191–197 (2018).
Gimenez-Cassina, A. et al. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron 74, 719–730 (2012).
Lian, X. Y., Khan, F. A. & Stringer, J. L. Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J. Neurosci. 27, 12007–12011 (2007).
McDaniel, S. S., Rensing, N. R., Thio, L. L., Yamada, K. A. & Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 52, e7–e11 (2011).
Warren, E. C. et al. Decanoic acid inhibits mTORC1 activity independent of glucose and insulin signaling. Proc. Natl Acad. Sci. USA 117, 23617–23625 (2020).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Koh, M. Y., Lim, K. S., Fong, S. L., Khor, S. B. & Tan, C. T. Impact of COVID-19 on quality of life in people with epilepsy, and a multinational comparison of clinical and psychological impacts. Epilepsy Behav. 117, 107849 (2021).
Kobow, K. et al. Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta Neuropathol. 126, 741–756 (2013).
Augustin, K. et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 17, 84–93 (2018).
Elamin, M., Ruskin, D. N., Sacchetti, P. & Masino, S. A. A unifying mechanism of ketogenic diet action: the multiple roles of nicotinamide adenine dinucleotide. Epilepsy Res. 167, 106469 (2020).
Garriga-Canut, M. et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat. Neurosci. 9, 1382–1387 (2006).
Koenig, J. B. et al. Glycolytic inhibitor 2-deoxyglucose prevents cortical hyperexcitability after traumatic brain injury. JCI Insight 5, e126506 (2019).
Schoeler, N. E. et al. K.Vita: a feasibility study of a blend of medium chain triglycerides to manage drug-resistant epilepsy. Brain Commun. 3, fcab160 (2021).
Sandau, U. S. et al. Transient use of a systemic adenosine kinase inhibitor attenuates epilepsy development in mice. Epilepsia 60, 615–625 (2019).
Masino, S. A. et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J. Clin. Invest. 121, 2679–2683 (2011).
Lusardi, T. A. et al. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 99, 500–509 (2015).
Toti, K. S., Osborne, D., Ciancetta, A., Boison, D. & Jacobson, K. A. South (S)- and North (N)-methanocarba-7-deazaadenosine analogues as inhibitors of human adenosine kinase. J. Med. Chem. 59, 6860–6877 (2016).
Schidlitzki, A. et al. Proof-of-concept that network pharmacology is effective to modify development of acquired temporal lobe epilepsy. Neurobiol. Dis. 134, 104664 (2020).
Welzel, L. et al. Network pharmacology for antiepileptogenesis: tolerability and neuroprotective effects of novel multitargeted combination treatments in nonepileptic vs. post-status epilepticus mice. Epilepsy Res. 151, 48–66 (2019).
Matos, M. et al. Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol. Psychiatry 78, 763–774 (2015).
Matos, M. et al. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia 60, 702–716 (2012).
Simeone, T. A., Simeone, K. A., Stafstrom, C. E. & Rho, J. M. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 133, 233–241 (2018).
Erecinska, M., Nelson, D., Daikhin, Y. & Yudkoff, M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J. Neurochem. 67, 2325–2334 (1996).
Buchhalter, J. R. et al. The relationship between D-beta-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open 2, 317–321 (2017).
Won, Y. J., Lu, V. B., Puhl, H. L. III & Ikeda, S. R. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci. 33, 19314–19325 (2013).
Kim, D. Y. et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 78, 77–87 (2015).
Puchalska, P. & Crawford, P. A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 (2017).
Moller, N. Ketone body, 3-hydroxybutyrate: minor metabolite–major medical manifestations. J. Clin. Endocrinol. Metab. 105, dgaa370 (2020).
Elinder, F. & Liin, S. I. Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Front. Physiol. 8, 43 (2017).
Bordoni, A., Di Nunzio, M., Danesi, F. & Biagi, P. L. Polyunsatured fatty acids: from diet to binding to ppars and other nuclear receptors. Genes Nutr. 1, 95–106 (2006).
Simeone, T. A., Matthews, S. A., Samson, K. K. & Simeone, K. A. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp. Neurol. 287, 54–64 (2017).
Bromfield, E. et al. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 12, 187–190 (2008).
Sarmento Vasconcelos, V. et al. Polyunsaturated fatty acid supplementation for drug-resistant epilepsy. Cochrane Database Syst. Rev. 17, CD011014 (2016).
Chang, P. et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 139, 431–443 (2016).
Loscher, W. & Schmidt, D. Epilepsy: perampanel–new promise for refractory epilepsy? Nat. Rev. Neurol. 8, 661–662 (2012).
Han, F. Y. et al. Dietary medium chain triglycerides for management of epilepsy: new data from human, dog, and rodent studies. Epilepsia 62, 1790–1806 (2021).
Willis, S., Stoll, J., Sweetman, L. & Borges, K. Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models. Neurobiol. Dis. 40, 565–572 (2010).
Calvert, S., Barwick, K., Par, M., Ni Tan, K. & Borges, K. A pilot study of add-on oral triheptanoin treatment for children with medically refractory epilepsy. Eur. J. Paediatr. Neurol. 22, 1074–1080 (2018).
Kumar, M. G. et al. Altered glycolysis and mitochondrial respiration in a zebrafish model of dravet syndrome. eNeuro 3, ENEURO.0008-16.2016 (2016).
Ibhazehiebo, K. et al. A novel metabolism-based phenotypic drug discovery platform in zebrafish uncovers HDACs 1 and 3 as a potential combined anti-seizure drug target. Brain 141, 744–761 (2018).
Ibhazehiebo, K., Rho, J. M. & Kurrasch, D. M. Metabolism-based drug discovery in zebrafish: an emerging strategy to uncover new anti-seizure therapies. Neuropharmacology 167, 107988 (2020).
Banerji, R. et al. Enhancing glucose metabolism via gluconeogenesis is therapeutic in a zebrafish model of Dravet syndrome. Brain Commun. 3, fcab004 (2021).
Koveal, D., Diaz-Garcia, C. M. & Yellen, G. Fluorescent biosensors for neuronal metabolism and the challenges of quantitation. Curr. Opin. Neurobiol. 63, 111–121 (2020).
Loscher, W. & Schmidt, D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 52, 657–678 (2011).
Boison, D. The biochemistry and epigenetics of epilepsy: focus on adenosine and glycine. Front. Mol. Neurosci. 9, 26 (2016).
Acknowledgements
J.M.R. has received funding from the Canadian Institutes of Health Research and the NIH (R21 NS104513), and D.B. has received funding from the NIH (R01NS103740, R01NS065957) and a CURE Epilepsy Catalyst Award. The authors thank R. Tobias for editorial assistance.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.M.R has been a paid consultant to Aquestive Pharmaceuticals, Danone Nutricia, Mallinckrodt, Eisai Pharma and Zogenix, and has served on the Scientific Advisory Board of The Charlie Foundation for Ketogenic Therapies (Santa Monica, CA, USA). D.B. is a co-founder of PrevEp and J.M.R. is the Chief Medical Officer for Path Therapeutics.
Peer review
Peer review information
Nature Reviews Neurology thanks C. Juhasz and K. Borges for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rho, J.M., Boison, D. The metabolic basis of epilepsy. Nat Rev Neurol 18, 333–347 (2022). https://doi.org/10.1038/s41582-022-00651-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00651-8
This article is cited by
-
Clinical and electroencephalogram characteristics of methylmalonic acidemia with MMACHC and MUT gene mutations
BMC Pediatrics (2024)
-
mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment
Nature Reviews Neuroscience (2024)
-
Simultaneous high-resolution whole-brain MR spectroscopy and [18F]FDG PET for temporal lobe epilepsy
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Genomic evidence for the suitability of Göttingen Minipigs with a rare seizure phenotype as a model for human epilepsy
neurogenetics (2024)
-
Systematic Analysis of the Relationship Between Elevated Zinc and Epilepsy
Journal of Molecular Neuroscience (2024)