Abstract
Cancer immunotherapy using monoclonal antibodies targeting immune checkpoints has undoubtedly revolutionized the cancer treatment landscape in the last decade. Immune checkpoint inhibitors can elicit long-lasting, previously unheard-of responses in a number of tumor entities. Yet, even in such tumors as metastatic melanoma and non-small cell-lung cancer, in which immune checkpoint inhibition has become the first-line treatment of choice, only a minority of patients will benefit considerably from these treatments. This has been attributed to a number of factors, including an immune-suppressive tumor microenvironment (TME). Using different modalities to break these barriers is of utmost importance to expand the population of patients that benefit from immune checkpoint inhibition. The multifunctional cytokine transforming growth factor-β (TGF-β) has long been recognized as an immune-suppressive factor in the TME. A considerable number of drugs have been developed to target TGF-β, yet most of these have since been discontinued. The combination of anti–TGF-β agents with immune checkpoint inhibitors now has the potential to revive this target as a viable immunomodulatory therapeutic approach. Currently, a limited number of small molecular inhibitor and monoclonal antibody candidates that target TGF-β are in clinical development in combination with the following immune checkpoint inhibitors: SRK 181, an antibody inhibiting the activation of latent TGF-β1; NIS 793, a monoclonal antibody targeting TGF-β; and SHR 1701, a fusion protein consisting of an anti-PD-L1 monoclonal antibody fused with the extracellular domain of human TGF-β receptor II. Several small molecular inhibitors are also in development and are briefly reviewed: LY364947, a pyrazole-based small molecular inhibitor of the serine-threonine kinase activity of TGFβRI; SB-431542, an inhibitor targeting several TGF-β superfamily Type I activin receptor-like kinases as well as TGF-β1-induced nuclear Smad3 localization; and galunisertib, an oral small molecular inhibitor of the TGFβRI kinase. One of the most advanced agents in this area is bintrafusp alfa, a bifunctional fusion protein composed of the extracellular domain of TGF-β receptor II fused to a human IgG1 mAb blocking PD-L1. Bintrafusp alfa is currently in advanced clinical development and as an agent in this space with the most clinical experience, is a focused highlight of this review.
Similar content being viewed by others
Simultaneous targeting of the PD-1/PD-L1 pathway and TGF-β can be done with maturing evidence of clinical activity. |
Targeting the PD-1/PD-L1 pathway and TGF-β can be accomplished without prohibitive safety concerns. |
Biomarker-driven approaches under development may help ascertain which patient population will derive maximal benefit from dual PD-1/PD-L1 pathway and TGF-β blockade. |
1 Background
1.1 Importance of the PD-1/PD-L1 Pathway
Over the past two decades, tumor immunobiologists have learned how the up-regulation of inhibitory receptor axes, such as cytotoxic T lymphocyte antigen 4 (CTLA-4)–CD28 and programmed cell death 1 (PD-1)–programmed cell death 1 ligand 1 (PD-L1), is an integral component of tumor immune escape, chemotherapy resistance, and disease progression [1]. Clinically, it is no secret that these discoveries have been revolutionary for the treatment of cancer and our understanding of intrinsic immune regulation. Following the approval of ipilimumab in March 2011 for metastatic melanoma [2], the landscape in which we have managed patients with advanced cancer has forever shifted. The magnitude of this paradigm change was punctuated by the 2018 Nobel Prize in Physiology or Medicine, awarded to James P. Allison and Tasuku Honjo for their discoveries leading to cancer treatments by way of suppressing negative immunomodulation [1]. In the years since the first PD-[L]1 inhibitor approval on September 4, 2014, there have been over 70 Biologic Licensing Applications (BLAs) for anti-PD-1- and anti-PD-L1-blocking antibodies approved [3]. The growing relevance of checkpoint inhibitors cannot be understated as they continue to change clinical practice and lead to the unprecedented extension of patient survival [4]. However, this story is far from over. As additional cancer and treatment-line indications are evaluated, it has become clear these agents have limits, often hampered by a variety of resistance mechanisms, including insufficient tumor immunogenicity, MHC dysfunction, T-cell exhaustion, resistance to secondary cytokines such as interferon (IFN)-γ signaling, and barriers on entering the immunosuppressive tumor microenvironment (TME) [5, 6].
1.2 Importance of the Transforming Growth Factor β (TGF-β) Pathway
A central factor underpinning tumor immune resistance is local immunosuppressive cytokines. A primary target in this space is transforming growth factor β (TGF-β). TGF-β is a 25-kDa dimeric protein [7], composed of two subunits, and is a multifunctional cytokine belonging to the transforming growth factor superfamily. This large superfamily of proteins include a substantial variety of protein families, such as bone morphogenetic proteins (BMPs), growth differentiation factors (GDFs), glial-derived neurotrophic factors (GDNFs), activins, inhibins, etc. In addition to this wide network are three different mammalian isoforms of TGF-β (TGF-β1, TGF-β2, TGF-β3), all of which function through the same receptor signaling pathways. Polypeptides from the TGF-β family were first isolated in the 1970s by de Larco and Todaro and were initially named as the sarcoma growth factor (SGF) as they could provoke the malignant transformation of rat kidney fibroblasts [8]. By the 1980s, Roberts and Sporn further described TGF-β as capable of inducing fibroblast growth and collagen production. Other groups around this time also identified TGF-β as having a dual role in its ability to inhibit cell proliferation as well [9]. Over the subsequent decades, we have learned of the numerous cellular and biological functions of the TGF-β superfamily, including regulation of cell proliferation, apoptosis, differentiation, and migration; embryonic patterning; stem cell maintenance; immune regulation; bone formation; and tissue remodeling and repair [10,11,12,13,14].
TGF-β1, a primary focus of this review, is composed of a latency-associated peptide (LAP) and a mature TGF-β1, which form homodimers via disulfide bonds. These homodimers then noncovalently associate as the small latent TGF-β1 complex (SLC). This secreted complex then covalently associates with a latent TGF-β binding protein (LTBP), thus creating a tripartite complex known as the large latent complex (LLC). The LLC is then sequestered within the extracellular matrix (ECM), which in turn functions as an ECM reservoir of TGF-β. Sequestration of latent TGF-β in the ECM is crucial for proper mobilization of the latent cytokine and its activation [15,16,17,18] (Fig. 1).
TGF-β precursor within the endoplasmic reticulum undergoes dimerization via disulfide bonds. These homodimers are cleaved by furin proteases into the small latent complex (SLC). This complex then associates with the latent TGF-β binding protein (LTBP) forming the large latent complex (LLC) before being secreted into the local extracellular environment. The LLC is then sequestered within the local extracellular matrix (ECM) where it acts as a reservoir for mature TGF-β. The sequestered mature TGF-β is released by proteases resulting in signaling through the TGF-β receptor inducing SMAD2/3 phosphorylation, followed by association with SMAD4 into a transcription complex. Furin cleavage and the non-covalent nature of the SLC are not visually represented
A growing body of evidence reveals that TGF-β1 can be activated by a variety of factors within the extracellular compartment, including plasmin, matrix metalloproteinases (MMPs), thrombospondin-1, lowered pH, and reactive oxygen species. Notably, TGF-β can also be activated by specific integrins that bind the Arg-Gly-Asp (RGD) sequence of LAPs. The integrin-RGD binding in turn results in a contractile-force-dependent conformational change of the latent complex, which releases a now-activated TGF-β. Furthermore, in proximity to the new, active TGF-β are a number of soluble extracellular agonists and antagonists that further complicate the temporal and spatial access of the ligands to receptors [17, 19,20,21,22,23,24].
TGF-β signaling involves three parallel pathways (BMP, TGF-β, and activin pathways), which converge through the canonical SMAD pathway that controls the expression of hundreds of genes, and several noncanonical pathways that regulate cell polarity, the cytoskeleton, and microRNA maturation [25]. Under normal homeostasis, TGF-β functions as a tumor suppressor, which can both induce apoptosis in pre-malignant cells and inhibit proliferation of cancerous cells. Under specific circumstances in which a tumor has inactivated the tumor-suppressive effects of TGF-β, either by a loss of specific downstream pathway signaling or a rewiring of this signaling, TGF-β can become a factor driving tumor progression. This co-option of TGF-β can be further skewed, wherein tumor-derived TGF-β can induce tumorigenic and pro-metastatic responses in cancer cells as well as the surrounding stroma, including the formation of an immune-suppressive TME [10, 26,27,28]. These biological changes can result clinically in more aggressive tumors, wherein reduced TGF-βRII expression in human non-small-cell lung cancer (NSCLC) leads to increased peri-tumor inflammation at least partially mediated by increased TGF-β1 expression [29]. We are also aware that TGF-β can induce chemoresistance by way of tumor quiescence, and this effect can be reversed or prevented by way of TGF-β inhibition [30].
As a result of the role TGF-β plays in both tumor propagation and metastasis, there has been interest in combining TGF-β blockade with additional immunotherapeutic approaches. This includes a significant interest in concurrent CTLA-4 or PD-1/PD-L1 blockade [31,32,33], as well as combinations with immunocytokines [34], cytokine/chemokine blockade [35, 36], oncolytic viruses [37], autologous tumor vaccination [38], and adoptive cell therapy [39]. The majority of these are outside the scope of this focused review.
1.3 Concentration of TGF-β Sequestration in Tumor Microenvironment (TME)
As tumors progress, they will typically generate and secrete their own TGF-β in an autocrine fashion. The TGF-β produced is sequestered as the LLC, which binds to local proteins within the ECM, predominantly fibrillin and fibronectin [16]. This ECM deposition serves as an abundant TGF-β reservoir impacting not only the tumor itself, but the local TME—inhibiting cell adhesion, inducing immunosuppression as well as angiogenesis, and lastly completing the cycle wherein further tumor-mediated or tumor-associated cell mediated degradation of the local ECM releases sequestered TGF-β and propagates the metastatic process. The latent TGF-β complex also binds glycoprotein A repetitions predominant (GARP), which is a transmembrane protein abundantly expressed on regulatory T cells and platelets [40]. GARP has been shown to play a central role in peripheral tolerance of T regulatory cells, as well as a source for ample TGF-β in the local microenvironment. This tumor-derived TGF-β not only drives the formation of cancer-associated fibroblasts [41], inhibits natural killer (NK) cells and dendritic (DC) cells [42, 43], but also serves to polarize macrophages into tumor-associated macrophages (TAMs) [44]. In addition, TGF-β is capable of impairing adaptive antitumor immunity through the direct inhibition of clonal expansion and cytotoxicity of CD8+ cytotoxic T cells [45, 46]. Lastly, TGF-β can induce the expression of Foxp3, which confers a regulatory and immunosuppressive phenotype [47]. Compounding this cycle, the GARP promoter has a binding site for FoxP3, which could in turn lead to further GARP expression and TGF-β sequestration to the local TME [16, 40, 48].
Previous studies have suggested that pan-inhibition of TGF-β may help overcome resistance to immune checkpoint blockade, but inhibitors blocking all three isoforms proved to be either too toxic for clinical use—often hindered by dose-limiting cardiotoxicities—or failed to show significant clinical activity despite promising preclinical evidence [49,50,51,52,53]. Several animal models and studies on loss-of-function mutations in humans of TGF-β2 and TGF-β3 isoforms suggest these isoforms may play vital homeostatic roles in cardiac function [51, 54,55,56,57]. This has led to dedicated interest in blocking the TGF-β1 isoform, as this appears to be the driver of immune resistance within the TME [58].
2 Preclinical and Early Phase Data
Several agents targeting TGF-β have been evaluated with mixed success, including several approaches using neutralizing antibodies, ligand traps, small-molecule inhibitors, and antisense oligonucleotides. Herein, we highlight eight agents that have shown promising activity.
2.1 SRK-181
The agent SRK-181 is a high-affinity, fully humanized monoclonal antibody that inhibits latent TGF-β1 activation. Preclinical work has displayed little to no binding to latent TGF-β2 and TGF-β3 isoforms or to active TGF-β growth factors [59]. In mouse tumor models (bladder, melanoma, and breast cancer), SRK-181 (in combination with anti-PD1 therapy) overcame primary anti-PD-1 resistance and showed survival benefit [58]. This has led to an ongoing multicenter, open-label, phase I trial of SRK-181 (DRAGON trial, ClinicalTrials.gov identifier NCT04291079), which evaluates SRK-181 alone or in combination with anti-PD-L1 inhibition in patients with locally advanced or metastatic solid tumors. One arm of this study involves assessing patients who have had prior anti-PD-1/PD-L1 therapy and are considered ‘nonresponders’ to assess whether adding SKR-181 can overcome primary anti-PD-1 resistance [60].
2.2 NIS 793
NIS793 (formerly XPA-42-068) is a pan anti-TGF-β-neutralizing antibody that has shown preclinical activity in xenograft models of pharyngeal carcinoma and squamous cell carcinoma [61, 62]. NIS793 was initially accessed across 120 participants in a phase I/Ib study (NCT02947165) in combination with spartalizumab (PDR001, an anti-PD-1 antibody) in patients with locally advanced or metastatic solid tumors. Interim results showed the agent was well tolerated, with 11% of patients experiencing a treatment-related adverse event (TRAE), the most common being rash (3%). Some clinical activity was noted, with two microsatellite-stable colorectal cancer patients achieving a partial response (PR) [63]. The antibody is currently being tested in a phase II clinical trial for patients with metastatic pancreatic ductal adenocarcinoma in combination with gemcitabine/nab-paclitaxel chemotherapy, as well as a separate arm including spartalizumab (NCT04390763) [64].
2.3 SHR 1701
An agent largely investigated in China is SHR-1701; this bispecific antibody is an anti-PD-L1 monoclonal antibody fused with the N-terminal-truncated extracellular domain of TGF-β receptor II (TGFβRII) [65]. This agent is biologically similar to another agent, bintrafusp alfa, discussed later in this review. The fused TGFβRII component functions as a TGF-β ‘trap,’ binding TGF-β within the TME. SHR-1701 is being investigated in 19 different phase I and phase II clinical trials (registered on ClinicalTrials.gov as of September 16, 2021) across a number of locally advanced and metastatic solid tumors. Of the data reported, the agent appears to be well tolerated with rare dose-limiting toxicity (DLT), including an incident of immune-mediated pneumonitis in a NSCLC expansion cohort [66], as well as a 46.9% reported incidence of immune-related adverse events across 49 patients with varying tumor types [67].
2.4 LY364947
LY364947 is a pyrazole-based small molecular inhibitor capable of inhibiting the serine-threonine kinase activity of TGFβRI. In several preclinical models, LY364947 decreased the resistance of glioblastoma-initiating cells [68], the MDA-MB-231 breast cancer cell line [69], and several non-small lung cancer cell lines (NCI-H1299, A549 and murine Lewis lung cancer cells) to radiotherapy [70, 71]. This observation is suggested to be in part mediated through attenuation of the DNA damage response pathway by TGFβRI inhibition. While there appears to be some promising preclinical data, no active trials are currently underway.
2.5 SB-431542
Another small molecular inhibitor, SB-431542, targets several TGF-β superfamily type I activin receptor-like kinases, including ALK4, ALK5, and ALK7, as well as subsequent TGFβ1–induced nuclear Smad3 localization. When tested with in vitro models, SB-431542 suppressed TGFβ-induced growth stimulation of MG63 osteosarcoma cells. While no active clinical trials exist for this inhibitor, SB-431542 has found renewed utility in preclinical stem cell differentiation protocols [71].
2.6 Galunisertib (LY2157299)
An agent with substantial pre-clinical evaluation is galunisertib, an oral small molecular inhibitor of the TGFβRI kinase which downregulates the phosphorylation of SMAD2. This agent has been studied in several disease states, including myelodysplastic syndrome where galunisertib decreased anemia in a TGF-β overexpressing transgenic mouse model of bone marrow failure [72, 73]. Galunisertib has also displayed antitumor activity across several xenograft models of breast, colon, lung, and hepatocellular carcinoma [71]. This preclinical activity led to a first-in-human dose-finding study in 65 patients with progressive malignancies [74]. This study included two arms, one for dose escalation and then a second that evaluated galunisertib in combination with standard clinical doses of lomustine. As a monotherapy, 16.6% (5/30) of evaluable galunisertib-treated patients experienced either a complete or partial response (CR or PR). Safety was assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 and galunisertib was evaluated as safe, with no cardiac adverse events and only three patients (7.7%) of the monotherapy arm experiencing grade 3 or 4 toxicities that were considered possibly drug related. One possible DLT was noted for grade 4 thrombocytopenia. A subsequent randomized phase II study of galunisertib involving 158 patients was completed; this involved three arms: galunisertib monotherapy (n = 39), galunisertib and lomustine (n = 79), or lomustine and placebo (n = 40) [75]. This too was a negative study where the combination of galunisertib and lomustine failed to demonstrate an improvement in overall survival (OS) relative to lomustine + placebo, with similar efficacy outcomes across all three arms. Another study from 2019 evaluated galunisertib in the second-line for patients with hepatocellular carcinoma [76]. Notably, OS was longer in AFP responders (> 20% decrease from baseline) compared with non-responders (21.5 months vs 6.8 months), and longer in TGF-β1 responders (> 20% decrease from baseline) compared with non-responders. The most common grade 3/4 TRAE were neutropenia (n = 4), as well as fatigue, anemia, hyperbilirubinemia, hypoalbuminemia, and embolism (each, n = 2). Most recently, a two-part, single-arm, multinational, phase Ib study was conducted of galunisertib co-administered with the anti-PD-L1 mAb, durvalumab, in patients with recurrent/refractory metastatic pancreatic cancer. No DLTs were recorded. Among 32 patients treated with galunisertib, one patient had PR, seven had stable disease (SD), 15 had objective progressive disease (PD), and nine were not evaluable. Disease control rate was 25.0%. Median OS and progression-free survival (PFS) were 5.72 months (95% CI 4.01–8.38) and 1.87 months (95% CI 1.58–3.09), respectively [77].
2.7 Vactosertib (TEW-7197)
Vactosertib (TEW-7197) is another selective small molecule inhibitor. This agent targets the adenosine-5-triphosphate binding site of TGFβR1, in turn inhibiting phosphorylation of the Smad2 and Smad3 proteins, the key mediators in TGF-β downstream signaling. Vactosertib safety, efficacy, and association with TGF-β response signatures were evaluated in patients with advanced solid tumors, identifying a response signature associated with poor prognosis. In a phase I modified 3 + 3 dose-escalating study of vactosertib, patients (n = 17) who received ≥ 140 mg achieved SD (35.3%) and had higher TGF-β response signatures than those with PD. Vactosertib was safe and well tolerated, and maximum tolerated dose was not determined. The most common TRAE was fatigue, while abdominal pain, AST elevation, and pulmonary edema occurred in one patient.
3 Bintrafusp alfa
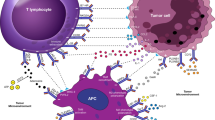
Bintrafusp alfa (formerly GSK-4045154, M7824, and MSB0011359C) is a first-in-class investigational bifunctional fusion protein designed to block TGF-β and PD-L1. The protein is composed of the extracellular domain of the TGF-βRII receptor, functioning here as a TGF-β ‘trap.’ This TGF-β trap is fused via a linker to the C-terminus of each heavy chain of an IgG1 antibody blocking PD-L1 (anti-PD-L1). As a result, bintrafusp alfa is designed to target tumors via first localization of the target drug, by way of anti-PD-L1 inhibition, with the simultaneous inhibition of two key mechanisms of immunosuppression in the TME [78,79,80,81] (Fig. 2). This proposed mechanism of action and drug localization was assessed by radiolabeling bintrafusp alfa with zirconium-89 (89Zr) and evaluating this radiolabeled conjugate in a PD-L1/TGF-β-positive murine breast cancer model (EMT-6). In this study, nanomolar affinities for PD-L1 were achieved with 89Zr-Df-bintrafusp alfa, suggesting the in vivo distribution patterns of bintrafusp alfa are driven by its PD-L1 binding arm [82].
In preclinical mouse tumor models, bintrafusp alfa showed greater antitumor activity versus anti–PD-L1 or anti–TGF-β treatment alone, supporting the biodistribution noted in radiolabeling studies. Treatment with bintrafusp alfa resulted in superior tumor regression at day 24 compared with treatment with either anti–PD-L1 or the trap control (both of which also showed partial antitumor activity). They also noted improved antitumor activity in mouse models of other solid tumors, including orthotopic breast models, colorectal cancer, and subcutaneous tumors. In addition, treatment with bintrafusp alfa resulted in significantly reduced cancer-associated fibroblast activity with reduced α-SMA expression relative to isotype control or anti–PD-L1 monotherapy and was shown to also reduce fibrosis. This suggests that with the use of bintrafusp alfa and the reduction in peri-tumor fibrosis, we may be able to help revert local drug resistance, increase antitumor activity, and improve the potential for synergy with combination therapies otherwise impeded by the TME. This was subsequently evaluated: bintrafusp alfa was combined with radiation therapy, which showed enhanced antitumor activity in preclinical mouse tumor models, whereas the combination of bintrafusp alfa with radiotherapy resulted in significantly reduced tumor volume and tumor weight relative to bintrafusp alfa or radiotherapy alone as well as a significantly increased frequency of IFN-γ-producing CD8+ T cells and the reduction in gene expression of epithelial-mesenchymal transition (EMT), vascular endothelial growth factor (VEGF) pathway, and radiation therapy (RT)–induced fibrosis gene-signatures [79].
Paralleling this work, Knudson et al. demonstrated that bintrafusp alfa sequesters murine TGF-β1 in vitro and in vivo. In addition, bintrafusp alfa can both prevent the initiation of, and significantly decrease existing TGF-β signaling, particularly in the TME [83]. They demonstrated that bintrafusp alfa reduces plasma TGF-β1, binds to PD-L1 in the tumor, and decreases TGF-β-induced signaling in the TME in mice. In murine breast and colon carcinoma models, bintrafusp alfa decreased both tumor burden and increased overall survival when compared with TGF-β neutralization alone. Bintrafusp alfa treatment promoted CD8+ T cell and NK cell activation, and both of these immune populations were required for optimal bintrafusp alfa-mediated tumor control. Bintrafusp alfa was superior to TGF-β- or PD-L1-targeted therapies when in combination with a therapeutic cancer vaccine. These findings demonstrate the value of using bintrafusp alfa to simultaneously target TGF-β and PD-L1/PD-1 immunosuppressive pathways to promote antitumor responses and efficacy. The studies also support the potential clinical use of bintrafusp alfa as a monotherapy or in combination with other immunotherapies, such as therapeutic cancer vaccines, including for patients who have progressed on PD-L1/PD-1 checkpoint blockade therapies [83].
Extending the potential synergy of therapeutic vaccines, Rumfield et al. investigated bintrafusp alfa in combination with a liposomal-based human papillomavirus (HPV) therapeutic vaccine consisting of an immune-activating cationic lipid (R-DOTAP) and HLA-unrestricted HPV16 peptides [84]. This study tested a syngeneic mouse model of a murine lung carcinoma cell line (TC-1) expressing HPV16 E6 and E7, devoid of PD-L1 expression to mimic a PD-L1 low patient population, with a combination of vaccine, bintrafusp alfa, and NHS-IL12 (an immunocytokine composed of two IL-12 heterodimers). HPV vaccine monotherapy generated HPV-specific T cells and antitumor activity in mice bearing TC-1 lung carcinomas, whereas bintrafusp alfa did not elicit antitumor effects or any increase in T cells in the TME. However, when combined with NHS-IL12, the three-agent therapy significantly reduced the rate of tumor growth and when compared with either therapy as a monotherapy, resulted in the lowest average tumor weight at the end of study. These results were then correlated with increases in T cells and T-cell clonality in the TME [84].
3.1 Clinical Data
Following promising preclinical data, early phase trials of bintrafusp alfa have started to reveal where it may be used alongside other agents in the burgeoning immunotherapy armamentarium to achieve antitumor synergy [80] (Table 1). Strauss et al. first evaluated bintrafusp alfa in a 3 + 3 dose-escalation phase I study to determine the safety and maximum tolerated dose (MTD). Nineteen heavily pretreated patients with ECOG 0–1 received bintrafusp alfa. Grade ≥ 3 TRAEs occurred in four patients (skin infection secondary to localized bullous pemphigoid, asymptomatic lipase increase, colitis with associated anemia, and gastroparesis with hypokalemia). In this study, MTD was not reached, and pharmacokinetic/pharmacodynamic studies revealed peripheral PD-L1 was saturated with > 80% occupancy throughout the dosing period. In addition, all released plasma TGF-β1, -β2, and -β3 isoforms were sequestered following bintrafusp alfa administration in a dose-dependent manner, with complete sequestration of all three isoforms found for the entire dosing period at doses > 1 mg/kg. At time of publication, the study reported efficacy across all dose levels, with a recommended phase II dose (RP2D) of 1200 mg every 2 weeks, including one ongoing confirmed CR (cervical cancer), two durable confirmed PRs (pancreatic cancer; anal cancer), one near-PR (cervical cancer), and two cases of prolonged SD (pancreatic cancer, carcinoid) [80].
Bintrafusp alfa was also studied in a separate phase I, open-label trial of advanced NSCLC that had progressed following platinum-based doublet therapy or platinum-based neoadjuvant or adjuvant treatment, as well as those who had not received prior immunotherapy [85]. Here, 80 patients were randomized at a one-to-one ratio to receive bintrafusp alfa at either 500 mg or at the RP2D of 1200 mg every 2 weeks. The median follow-up in this study was 51.9 weeks, with an overall response rate (ORR) of 25.0% in the RP2D cohort (10/40 patients). Notably, at the RP2D, patients with PD-L1-positive and PD-L1-high (≥ 80% expression on tumor cells) disease had ORRs of 36.0% (10/27 patients) and 85.7% (6/7 patients), respectively. We note in this study, given the patients receipt of prior therapy, it is unclear if the increase in PD-L1-positive responses seen were in part conditional on T-cell responses elicited following their prior systemic therapy. In this study, PD-L1 status was obtained from fresh tumor biopsies within 28 days prior to first drug administration, and all patients were required to have been free of prior systemic treatment for a minimum of 28 days. The treatment was tolerated with 68.8% (55/80 patients) experiencing a TRAE (500 mg, 27/40; 1200 mg, 28/40 patients), of which the most common (experienced by ≥ 10% of patients) were pruritis (21.3%), maculopapular rash (18.8%), decreased appetite (12.5%), and asthenia (11.3%). By study close, 10% (8/80 patients) had a TRAE that led to treatment discontinuation, with no treatment-related deaths during the study [85]. This initial study in NSCLC led to a head-to-head trial of bintrafusp alfa versus pembrolizumab, named INTR@PID lung 037, as first-line treatment in patients with advanced NSCLC [86]. However, this latter trial was discontinued in January 2020 after review by an independent data monitoring committee, which showed the study was unlikely to meet its coprimary endpoints of PFS and OS. Several criticisms have risen with respect to the trial design, including it being an unblinded study and that the clinical investigators may have been largely unfamiliar with the side effect profile of bintrafusp alfa, potentially leading to early discontinuation [87].
Highlighting the broad potential for bintrafusp alfa across epithelial cancers, a separate phase I study evaluated bintrafusp alfa in Asian patients with biliary tract cancers (BTCs) who had progressed despite prior adjuvant or neoadjuvant chemotherapy [88]. In this study, bintrafusp alfa was administered at 1200 mg every 2 weeks until either confirmed PD, unacceptable toxicity, or trial withdrawal. Median follow-up time was 15.3 months, with a median duration of therapy of 8.9 months, and three patients who remained on active treatment for > 59.7 weeks. The ORR was 20%, with 7% (2/30 patients) experiencing a CR lasting > 12.5 months, 13% (4/30 patients) experiencing PRs, and 20% (6/30 patients) with SD. Similar to prior trials, the agent was generally well tolerated, with 37% (11/30 patients) experiencing a grade 3 or greater TRAE, with the most common (experienced by ≥ 10% of patients) being rash in 13% (4/30 patients) and elevated lipase in 10% (3/30 patients). However, the study did report three patient deaths possibly related to treatment: one septic shock event due to bacteremia, which led to death, as well as two cases of interstitial pneumonitis (ILD), which led to death—one of which occurred 6 months after the last bintrafusp alfa dose. The authors note these were the only cases of ILD across their entire phase I program evaluating bintrafusp alfa (NCT02699515 and NCT02517398; combined n = 689 as of August 24, 2019) [88]. A subsequent phase II trial (INTR@PID BTC 047, NCT03833661) for BTCs went on to evaluate bintrafusp alfa as second-line monotherapy for patients with locally advanced or metastatic biliary tract cancers who were ineligible for or for whom first-line platinum-based chemotherapy has failed. Final results showed signs of efficacy with a 10.1% ORR at 9 months of follow up, nearly double the 5.8% ORR of pembrolizumab monotherapy in a similar patient population [89, 90]. However, although single-agent activity was noted, this study did not meet its predefined endpoint. Until August 2021, bintrafusp alfa remained under investigation for BTCs as part of the phase II/III INTR@PID BTC 055 (NCT04066491) trial, evaluating front-line use of bintrafusp alfa in combination with gemcitabine and cisplatin [91]. However, this study was discontinued early following recommendations by the trial’s independent data monitoring committee, who concluded the trial was unlikely to meet its primary end point of OS [92].
More recently, bintrafusp alfa has been evaluated in HPV-associated malignancies [93]. These malignancies are viewed as those with a higher yield of response, as genome-wide association studies noted a relationship between the TGF-β pathway and cervical cancer as well as HPV-positive squamous cell carcinoma of the head and neck (SCCHN) [94]. Furthermore, TGF-β receptor I is significantly overexpressed in these cancers compared with benign tissue, and dysregulated TGF-β signaling has been associated with malignant progression of HPV-positive cervical dysplasia, as well as evidence HPV can mediate promotion of cervical cancer by attenuating TGF-βR1 signaling required for epithelial homeostasis at early stages of viral infection [94,95,96]. To assess whether this population of HPV+ malignancies may be uniquely susceptible to the tandem effects of bintrafusp alfa, a post-hoc analysis of bintrafusp alfa across a combined HPV+ population was performed. This analysis included those patients treated on a phase I, open-label trial of bintrafusp alfa with heavily pretreated advanced solid tumors (n = 43) as well as a phase II, single-center trial of patients with advanced HPV-associated cancers (n = 16). Those patients within the phase I dose-escalation trial received bintrafusp alfa once every 2 weeks at doses of 0.3–30 mg/kg, whereas those on the RP2D received bintrafusp alfa at 1200 mg every 2 weeks, for a combined population of 75 patients. Across this combined cohort of heavily pretreated patients with a median follow-up of 33 months, investigators found a confirmed ORR of 28.0% (n = 21, 4 CRs and 17 PRs), with three additional patients achieving a delayed PR, leading to a clinical response rate of 32.0% and the suggestion further studies in this population of HPV+ malignancies may be warranted. Notably, the median duration of response was 17.3 months, and the median OS was 21.3 months, with a 12-month OS rate of 59.7%. The TRAEs were similar to prior trials, with the most common being grade 1 pruritis in 25.3% of patients and grade 1 dermatitis acneiform. No treatment-related deaths occurred [93, 97].
A third trial evaluating bintrafusp alfa in 14 patients with HPV16+ relapsed or refractory advanced cancer has also been reported. This trial incorporated a triple combination of 1200 mg bintrafusp alfa every 2 weeks with M9241, an immunocytokine composed of IL-12 heterodimers fused to a monoclonal antibody targeting free DNA proximal to necrotic tumors [98], and PDS0101 (Versamune-HPV), a liposomal multipeptide therapeutic vaccine targeting HPV 16 E6/E7 [99]. With a median follow-up of 5 months, investigators reported one CR, and nine PRs with nine out of ten responses ongoing at time of data cut off. They noted of the 14 patients, six were checkpoint naive and eight had checkpoint refractory disease. Of those with checkpoint-naive disease, five of six (83%) experienced an objective response, whereas five of the eight patients (63%) with checkpoint refractory disease experienced an objective response. The treatment combination was largely well tolerated, with no treatment-related deaths and four grade 3 TRAEs (hematuria in two patients with cervical cancer and prior pelvic radiation as well as two patients with AST/ALT elevations).
These data highlight the potential applicability of bintrafusp alfa in a focal patient population as well as the novel toxicities seen as a result of TGF-β sequestration.
3.2 Side Effects
3.2.1 Overview
Several toxicities have been identified in TGF-β inhibitors, including bintrafusp alfa. A combined cohort of 606 patients across the phase I INTR@PID 001 and 008 studies in heavily pretreated solid tumors was presented at the 2021 ESMO annual meeting [100]. TRAEs of any grade occurred in 68.3% of patients (n = 414), with grade ≥ 3 TRAEs in 22.3% of patients (n = 135). Out of the 606 patients, 8.7% permanently discontinued (n = 53) treatment because of TRAEs. The most common adverse events included TGF-β inhibition-mediated skin adverse events (any grade: 11.9%, grade ≥ 3: 2.6%), immune-related adverse events (any grade: 23.3%, grade ≥ 3: 8.9%), anemia (any grade: 30.5%, grade ≥ 3: 18.0%), bleeding events (any grade: 39.3%, grade ≥ 3: 10.2%), and infusion-related reactions (any grade: 6.3%, grade ≥ 3: 0.2%). Notably, the most common skin adverse events were keratoacanthomas (KAs), typically in older, light-skinned patients with a history of sun-damage, and the most common bleeding event was epistaxis. In these trials, the eligibility criteria included an exclusion for bleeding diathesis or recent major bleeding. As the majority of reported bleeding events were mild to moderate mucosal bleeding; these were clinically manageable and resolved without the need for bintrafusp alfa discontinuation. One important difference in toxicity profile noted with bintrafusp alfa is the distinct lack of significant cardiac toxicity, a concern noted with prior pan-TGF-β inhibitors [53].
3.2.2 Bleeding
Although most of the reported bleeding events were low-grade mucosal bleeding (e.g., epistaxis, gingival bleeding), there are episodes of significant and at times life-threatening bleeding (e.g., gastrointestinal hemorrhage). Bleeding from TGF-β inhibitors was identified in early studies of fresolimumab, an engineered human monoclonal Ig that neutralizes the three major isoforms of TGF-β. Studies in fifteen patients with systemic sclerosis identified two cases of clinically significant gastrointestinal bleeding from gastric antral vascular ectasia, as well as three cases of gingival bleeding and/or epistaxis with two others reporting subconjunctival hemorrhage [101]. Three patients in a separate study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis developed grade ≥ 3 gingival bleeding [102].
In a phase I expansion cohort of patients with recurrent glioblastoma, six patients (17.1%) experienced gingival bleeding, whereas five patients (14.3%) experienced intratumoral or intracranial bleeding events in the setting of progressive disease. The intratumoral and intracranial bleeding events occurred between 2 and 17 days after their last dose of bintrafusp alfa, with two of the five patients concurrently receiving anticoagulation (for deep vein thrombosis prophylaxis and as maintenance following prior pulmonary embolism). Notably, all of these events occurred in new lesions attributed to progressive disease, and this rate of intracranial hemorrhage was similar to reported rates in patients with primary brain tumors receiving disease-directed treatment who are not on anticoagulation (2.6–13.6%) and are on anticoagulation (15.5–28.1%) [103, 104]. Of note, one of these intratumoral hemorrhage events did lead to a patient death and was assessed by investigators as treatment-related in conjunction with disease progression [81].
In a phase I study of bintrafusp alfa in Asian patients with advanced solid tumors, one patient with a pituitary gland tumor developed intralesional bleeding, which was attributed as probably related to treatment. Two other patients developed grade 3 upper gastrointestinal hemorrhage and pulmonary hemorrhage, respectively, although both were attributed as unrelated to treatment [105].
In an evaluation of bintrafusp alfa from phase I and II trials in cervical cancer, there were single reports of grade 3 treatment-related upper-gastrointestinal hemorrhage as well as hematuria [106]. This cohort was assessed in a larger data set of HPV-related malignancies, and across 59 patients, 38 patients (64.4%) experienced treatment-emergent bleeding, with nine patients (15.3%) experiencing grade 3 bleeding events [93].
A poster summarizing the safety profile of bintrafusp alfa (from the INTR@PID LUNG 024 study evaluating bintrafusp alfa in combination with chemotherapy) noted epistaxis in ~ 30 to 44% of patients experiencing treatment-emergent adverse events, depending on cohort reviewed, as well as ~ 33% of patients experiencing hemoptysis, with one noted as grade ≥ 3 [107].
At present, it remains unclear what the mechanism of toxicity is when TGF-β is inhibited. We know TGF-β does play a vital role in the homeostasis of the adult microvasculature as well as maintaining vascular barrier function and survival [13]. Similarly, we know TGF-β1 plays a key role in enhancing platelet aggregation through the activation and maintenance of the α11b/β3 fibrinogen receptor [108]. This would imply the possibility of a Glanzmann thrombasthenia–like bleeding phenomenon; however, platelet studies on patients with bleeding have not displayed marked deficits in function (internal data, includes samples from NCT02517398, pending publication). As TGF-β inhibitors move forward in their clinical application, it will be equally important to investigate the pathology of TGF-β-inhibitor–related bleeding adverse events.
3.2.3 Skin Changes
A separate but disruptive side effect noted with TGF-β inhibitors is skin toxicity, such as KAs, at times leading to drug discontinuation. In a phase I study of bintrafusp alfa monotherapy in Asian patients with BTCs, 2 of 30 patients developed KAs [88]. In a separate analysis of bintrafusp alfa monotherapy, dosed every 2 weeks, in HPV-related malignancies across two studies (NCT02517398 and NCT03427411), 12 patients (20.3%) experienced treatment-related skin lesions, of which ten (16.9%) were KAs, and another eight reported events of basal cell carcinoma, squamous cell carcinoma of the skin or lip, hyperkeratosis, and actinic keratosis. Notably, across these reported skin lesions, only four were reported as grade 3 in severity [93].
4 Ongoing Trials and Future Directions
As of September 2021, there are 42 active, ongoing, or completing trials evaluating bintrafusp alfa across a wide array of malignancies and in combination with a multitude of cancer-directed therapies, from traditional chemotherapeutics to radiation therapy to additional checkpoint inhibitors, cytokines, and vaccines (Table 2). Each of the trial experiences with bintrafusp alfa have revealed a subset of patients who experience durable clinical benefit with noted CR and PRs among each cohort. These experiences were seen across malignancies and irrespective of PD-L1 status, suggesting an opportunity to identify a predictive biomarker signature of response. Furthermore, as bintrafusp alfa has been well tolerated across studies, it remains as a readily available agent to include in combination trials—many of which are underway.
Clinical studies have demonstrated the safety and activity of therapeutic approaches simultaneously targeting the PD-1/PD-L1 pathway and TGF-β. Although several initial phase II studies of bintrafusp alfa have not met their prespecified primary endpoint or were deemed not likely to meet them, the future for combined targeting of these two pathways remains solid. Data with bintrafusp alfa in HPV-associated malignancies remain very promising. Additional understanding of the clinical implications for the complex biology of TGF-β in the TME, and which patients might benefit most, are being pursued by multiple groups.
References
Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–42.
Press Announcements > FDA approves new treatment for a type of late-stage skin cancer [Internet]. [cited 2021 Aug 5]. https://web.archive.org/web/20110327063147/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm1193237.htm.
Chang E, Pelosof L, Lemery S, Gong Y, Goldberg KB, Farrell AT, et al. Systematic review of PD-1/PD-L1 inhibitors in oncology: from personalized medicine to public health. Oncologist. 2021;26:e1786–99.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance mechanisms of anti-PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020;8:672.
Cormedi MCV, Van Allen EM, Colli LM. Predicting immunotherapy response through genomics. Curr Opin Genet Dev. 2021;66:1–9.
Herpin A, Lelong C, Favrel P. Transforming growth factor-β-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol. 2004;28:461–85.
Poniatowski LA, Wojdasiewicz P, Gasik R, Szukiewicz D. Transforming growth factor beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediat Inflamm. 2015;2015: 137823.
Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β—an excellent servant but a bad master. J Transl Med. 2012;10:1–24.
Lebrun J-J. The dual role of TGFβ in human cancer: from tumor suppression to cancer metastasis. ISRN Mol Biol. 2012;2012:1–28.
Papageorgis P. TGFβ signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015: 587193.
Han Z, Kang D, Joo Y, Lee J, Oh G-H, Choi S, et al. TGF-β downregulation-induced cancer cell death is finely regulated by the SAPK signaling cascade. Exp Mol Med. 2018;50:1–19.
Walshe TE, Saint-Geniez M, Maharaj ASR, Sekiyama E, Maldonado AE, D’Amore PA. TGF-β is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS ONE. 2009;4:1–16.
Moses HL, Roberts AB, Derynck R. The discovery and early days of TGF-β: a historical perspective. Cold Spring Harb Perspect Biol. 2016;8: a021865.
Horiguchi M, Ota M, Rifkin DB. Featured: Matrix control of transforming growth factor-β function. J Biochem. 2012;152:321.
Robertson IB, Rifkin DB. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol. 2016;8:21907–8.
Xu X, Zheng L, Yuan Q, Zhen G, Crane JL, Zhou X, et al. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018;6:1–31.
Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53.
Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28.
Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–24.
Wang M, Zhao D, Spinetti G, Zhang J, Jiang L-Q, Pintus G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-β1 (TGF-β1) and TGF-β1–type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–9.
Pozzi A, Zent R. TGF-β sequestration by mesangial cell integrin αvβ8: a novel mechanism of glomerular endothelial cell regulation. Am J Pathol. 2011;178:485–9.
Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. Latent TGF-β structure and activation. Nature. 2011;474:343–51.
Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 2015;47:54–65.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
Pickup MW, Owens P, Moses HL. TGF-β, bone morphogenetic protein, and activin signaling and the tumor microenvironment. Cold Spring Harb Perspect Biol. 2017;9: a022285.
David CJ, Massagué J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19:419–35.
Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50:924–40.
Malkoski SP, Haeger SM, Cleaver TG, Rodriguez KJ, Li H, Lu S-L, et al. Loss of transforming growth factor beta type II receptor increases aggressive tumor behavior and reduces survival in lung adenocarcinoma and squamous cell carcinoma. Clin Cancer Res. 2012;18:2173.
Brown JA, Yonekubo Y, Hanson N, Sastre-Perona A, Basin A, Rytlewski JA, et al. TGF-β-induced quiescence mediates chemoresistance of tumor-propagating cells in squamous cell carcinoma. Cell Stem Cell. 2017;21:650-664.e8.
Ungefroren H. Blockade of TGF-β signaling: a potential target for cancer immunotherapy? Expert Opin Ther Targets. 2019;23(8):679–93.
van den Bulk J, de Miranda NFCC, ten Dijke P. Therapeutic targeting of TGF-β in cancer: hacking a master switch of immune suppression. Clin Sci. 2021;135:35–52.
Bai X, Yi M, Jiao Y, Chu Q, Wu K. Blocking TGF-β signaling to enhance the efficacy of immune checkpoint inhibitor. Onco Targets Ther. 2019;12:9527.
Xu C, Marelli B, Qi J, Qin G, Yu H, Wang H, et al. NHS-IL12 and bintrafusp alfa combination therapy enhances antitumor activity in preclinical cancer models. Transl Oncol. 2021;16: 101322.
Horn LA, Riskin J, Hempel HA, Fousek K, Lind H, Hamilton DH, et al. Simultaneous inhibition of CXCR1/2, TGF-β, and PD-L1 remodels the tumor and its microenvironment to drive antitumor immunity. J Immunother Cancer. 2020;8(1):e000326.
Bialkowski L, Van der Jeught K, Bevers S, Tjok Joe P, Renmans D, Heirman C, et al. Immune checkpoint blockade combined with IL-6 and TGF-β inhibition improves the therapeutic outcome of mRNA-based immunotherapy. Int J Cancer. 2018;143:686–98.
Groeneveldt C, van Hall T, van der Burg SH, ten Dijke P, van Montfoort N. Immunotherapeutic potential of TGF-β inhibition and oncolytic viruses. Trends Immunol. 2020;41:406–20.
Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B, et al. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143:504–10.
Hartley J, Abken H. Chimeric antigen receptors designed to overcome transforming growth factor-β-mediated repression in the adoptive T-cell therapy of solid tumors. Clin Transl Immunol. 2019;8(6): e1064.
Metelli A, Salem M, Wallace CH, Wu BX, Li A, Li X, et al. Immunoregulatory functions and the therapeutic implications of GARP-TGF-β in inflammation and cancer. J Hematol Oncol. 2018;11:1–11.
Stuelten CH, Busch JI, Tang B, Flanders KC, Oshima A, Sutton E, et al. Transient tumor-fibroblast interactions increase tumor cell malignancy by a TGF-Beta mediated mechanism in a mouse xenograft model of breast cancer. PLoS ONE. 2010;5(3): e9832.
Marcoe JP, Lim JR, Schaubert KL, Fodil-Cornu N, Matka M, McCubbrey AL, et al. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat Immunol. 2012;13:843–50.
Novitskiy SV, Pickup MW, Chytil A, Polosukhina D, Owens P, Moses HL, et al. Deletion of TGF-β signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol. 2012;92:641–51.
Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:58.
Gorelink L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–22.
Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80.
Em S. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45.
Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D, et al. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13439–44.
Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, et al. Phase I study of GC1008 (Fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE. 2014;9(3): e90353.
Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39:916–24.
Frangogiannis NG. The role of transforming growth factor (TGF)-β in the infarcted myocardium. J Thorac Dis. 2017;9:S52-63.
Parichatikanond W, Luangmonkong T, Mangmool S, Kurose H. Therapeutic targets for the treatment of cardiac fibrosis and cancer: focusing on TGF-β signaling. Front Cardiovasc Med. 2020;7:34.
Mitra MS, Lancaster K, Adedeji AO, Palanisamy GS, Dave RA, Zhong F, et al. A potent pan-TGFβ neutralizing monoclonal antibody elicits cardiovascular toxicity in mice and cynomolgus monkeys. Toxicol Sci. 2020;175:24–34.
Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, et al. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659.
Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44:922.
González-Núñez M, Muñoz-Félix JM, López-Novoa JM. The ALK-1/Smad1 pathway in cardiovascular physiopathology. A new target for therapy? Biochim Biophys Acta Mol Basis Dis. 2013;1832:1492–510.
Schepers D, Tortora G, Morisaki H, MacCarrick G, Lindsay M, Liang D, et al. A mutation update on the LDS-associated genes TGFB2/3 and SMAD2/3. Hum Mutat. 2018;39:621–34.
Martin CJ, Datta A, Littlefield C, Kalra A, Chapron C, Wawersik S, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med. 2020;12:8456.
Welsh BT, Faucette R, Bilic S, Martin CJ, Schürpf T, Chen D, et al. Nonclinical development of SRK-181: an anti-latent TGFβ1 monoclonal antibody for the treatment of locally advanced or metastatic solid tumors. Int J Toxicol. 2021;40:226.
Yap TA, Barve MA, Gainor JF, Weekes CD, Bockorny B, Ju Y, et al. First-in-human phase 1 trial (DRAGON) of SRK-181, a potential first-in-class selective latent TGFβ1 inhibitor, alone or in combination with anti-PD-(L)1 treatment in patients with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl):TPS3146.
Bedinger D, Lao L, Khan S, Lee S, Takeuchi T, Mirza AM. Development and characterization of human monoclonal antibodies that neutralize multiple TGFb isoforms. MAbs. 2016;8(2):389–404.
Dodagatta-Marri E, Meyer DS, Reeves MQ, Paniagua R, To MD, Binnewies M, et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J Immunother Cancer. 2019;7:1–15.
Bauer TM, Lin C-C, Greil R, Goebeler M-E, Huetter-Kroenke ML, Garrido-Laguna I, et al. Phase Ib study of the anti-TGF-β monoclonal antibody (mAb) NIS793 combined with spartalizumab (PDR001), a PD-1 inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl):2509.
Grell P, Lin C-C, Milella M, Chee CE, Sivakumar S, Peltola KJ, et al. Phase II study of the anti-TGF-β monoclonal antibody (mAb) NIS793 with and without the PD-1 inhibitor spartalizumab in combination with nab-paclitaxel/gemcitabine (NG) versus NG alone in patients (pts) with first-line metastatic pancreatic ductal adenocarcinoma (mPDAC). J Clin Oncol. 2021;39(15_suppl):TPS4173.
Zhang J, Yi J, Zhou P. Development of bispecific antibodies in China: overview and prospects. Antib Ther. 2020;3:126–45.
Shi M, Chen J, Li K, Fang Y, Wen G, Li X, et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, for advanced NSCLC with EGFR mutations: Data from a multicenter phase 1 study. J Clin Oncol. 2021;39(15_suppl):9055.
Liu D, Gong J, Liu T, Li K, Yin X, Liu Y, et al. Phase 1 study of SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl):2503.
Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 2012;72:4119–29.
Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res. 2011;17:6754–65.
Du S, Bouquet S, Lo CH, Pellicciotta I, Bolourchi S, Parry R, et al. Attenuation of the DNA damage response by transforming growth factor-beta inhibitors enhances radiation sensitivity of non-small-cell lung cancer cells in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2015;91:91–9.
Teicher BA. TGFβ-directed therapeutics: 2020. Pharmacol Ther. 2021;217: 107666.
Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, Fazzari M, et al. Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71:955–63.
Santini V, Valcarcel D, Platzbecker U, Komrokji RS, Cleverly AL, Lahn MM, et al. Phase II study of the ALK5 inhibitor galunisertib in very low-, low-, and intermediate-risk myelodysplastic syndromes. Clin Cancer Res. 2019;25:6976–85.
Rodon J, Carducci MA, Sepulveda-Sánchez JM, Azaro A, Calvo E, Seoane J, et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21:553–60.
Brandes AA, Carpentier AF, Kesari S, Sepulveda-Sanchez JM, Wheeler HR, Chinot O, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016;18:1146.
Faivre S, Santoro A, Kelley RK, Gane E, Costentin CE, Gueorguieva I, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39:1468–77.
Melisi D, Oh DY, Hollebecque A, Calvo E, Varghese A, Borazanci E, et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother cancer. 2021;9(3): e002068.
David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C, et al. A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology. 2017;6(10): e1349589.
Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10:5488.
Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res. 2018;24:1287–95.
Khasraw M, Weller M, Lorente D, Kolibaba K, Lee CK, Gedye C, et al. Bintrafusp alfa (M7824), a bifunctional fusion protein targeting TGF-β and PD-L1: results from a phase I expansion cohort in patients with recurrent glioblastoma. Neuro-Oncol Adv. 2021;3:1–11.
Burvenich IJG, Goh YW, Guo N, Gan HK, Rigopoulos A, Cao D, et al. Radiolabelling and preclinical characterization of 89 Zr-Df-radiolabelled bispecific anti-PD-L1/TGF-βRII fusion protein bintrafusp alfa. Eur J Nucl Med Mol Imaging. 2021;48:3075–88.
Knudson KM, Hicks KC, Luo X, Chen J-Q, Schlom J, Gameiro SR. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018;7(5): e1426519.
Rumfield CS, Pellom ST, Maurice Y, Ii M, Schlom J, Jochems C. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J Immunother Cancer. 2020;8:612.
Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15:1210–22.
Ahn M-J, Barlesi F, Felip E, Garon E, Martin CM, Vokes E, et al. MO01.29 randomized, open-label study of bintrafusp alfa vs. pembrolizumab as first-line (1L) treatment in patients with PD-L1–expressing advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2021;16:S27–8.
Bintrafusp Alfa 037 Update - News | Merck KGaA, Darmstadt, Germany [Internet]. [cited 2021 Sep 29]. https://www.emdgroup.com/en/news/bintrafusp-alfa-037-update-20-01-2021.html.
Yoo C, Oh D-Y, Choi HJ, Kudo M, Ueno M, Kondo S, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020;8:564.
Ueno M, Chung HC, Nagrial A, Marabelle A, Kelley RK, Xu L, et al. Pembrolizumab for advanced biliary adenocarcinoma: results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol. 2018;29:viii210.
Reporting Topline Data for Bintrafusp Alfa as Second-Line Monotherapy Treatment | Merck KGaA, Darmstadt, Germany [Internet]. [cited 2021 Sep 29]. https://www.emdgroup.com/en/news/bintrafusp-topline-data-biliary-tract-cancer-16-03-2021.html.
Bintrafusp Alfa Monotherapy Showcases Efficacy, Durability in Second-Line Biliary Tract Cancer [Internet]. [cited 2021 Sep 29]. https://www.onclive.com/view/bintrafusp-alfa-monotherapy-showcases-efficacy-durability-in-second-line-biliary-tract-cancer.
Bintrafusp Alfa Update - News | Merck KGaA, Darmstadt, Germany [Internet]. [cited 2021 Sep 29]. https://www.emdgroup.com/en/news/bintrafusp-alfa-update-23-08-2021.html.
Strauss J, Gatti-Mays ME, Cho BC, Hill A, Salas S, Mcclay E, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer. 2020;8:1395.
Levovitz C, Chen D, Ivansson E, Gyllensten U, Finnigan JP, Alshawish S, et al. TGFβ receptor 1: an immune susceptibility gene in HPV-associated cancer. Cancer Res. 2014;74:6833.
Deng W, Sai WT, Kwok YK, Wong E, Xiao RH, Liu S, et al. Transforming growth factor beta1 promotes chromosomal instability in human papillomavirus 16 E6E7-infected cervical epithelial cells. Cancer Res. 2008;68:7200–9.
French D, Belleudi F, Mauro MV, Mazzetta F, Raffa S, Fabiano V, et al. Expression of HPV16 E5 down-modulates the TGFbeta signaling pathway. Mol Cancer. 2013;12:38.
Strauss J, Gatti-Mays M, Cho BC, Hill A, Salas S, McClay E, et al. Long-term follow-up of patients (pts) with human papillomavirus (HPV)eassociated malignancies treated with bintrafusp alfa, a bifunctional fusion protein targeting TGF-b and PD-L1. Ann Oncol. 2021;32(5_Suppl):957O.
Strauss J, Heery CR, Kim JW, Jochems C, Donahue RN, Montgomery AS, et al. First-in-human phase i trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin Cancer Res. 2019;25:99–109.
Strauss J, Floudas CS, Sater HA, Manu M, Lamping E, Francis DC, et al. Phase II evaluation of the triple combination of PDS0101, M9241, and bintrafusp alfa in patients with HPV 16 positive malignancies. J Clin Oncol. 2021;39(15_Suppl):2501.
Gulley JL, Lacouture M, Spira A, Mata HV, Yoo C, Cho BC, et al. Adverse event management during treatment with bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1: treatment guidance based on experience in clinical trials. Ann Oncol. 2021;31(5_Suppl):S1181.
Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795.
Vincenti F, Fervenza FC, Campbell KN, Diaz M, Gesualdo L, Nelson P, et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep. 2017;2:800.
Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI, et al. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017;129:3379–85.
Khoury MN, Missios S, Edwin N, Sakruti S, Barnett G, Stevens G, et al. Intracranial hemorrhage in setting of glioblastoma with venous thromboembolism. Neuro-oncology Pract. 2016;3:87–96.
Doi T, Fujiwara Y, Koyama T, Ikeda M, Helwig C, Watanabe M, et al. Phase I study of the bifunctional fusion protein bintrafusp alfa in Asian patients with advanced solid tumors, including a hepatocellular carcinoma safety-assessment cohort. Oncologist. 2020;25: e1292.
Strauss J, Braiteh FS, Calvo E, De MM, Cervantes A, Edenfield WJ, et al. Evaluation of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in cervical cancer: Data from phase 1 and phase 2 studies. J Clin Oncol. 2021;39(15_Suppl):5509.
Rolfo C, Greillier L, Veillon R, Badin F, Ghiringhelli F, Isambert N, et al. Abstract CT104: Bintrafusp alfa in combination with chemotherapy in patients with stage IV NSCLC: safety results of the INTR@PID LUNG 024 study. Cancer Res 2021;81:CT104.
Hoying JB, Yin M, Diebold R, Ormsby I, Becker A, Doetschman T. Transforming growth factor β1 enhances platelet aggregation through a non-transcriptional effect on the fibrinogen receptor. J Biol Chem. 1999;274:31008–13.
Cho BC, Daste A, Ravaud A, Salas S, Isambert N, McClay E, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in advanced squamous cell carcinoma of the head and neck: results from a phase I cohort. J Immunother Cancer. 2020;8(2): e000664.
Tan B, Khattak A, Felip E, Kelly K, Rich P, Wang D, et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients with Esophageal Adenocarcinoma: Results from a Phase 1 Cohort. Target Oncol. 2021;16(4):435–46.
Acknowledgments
Figures created with biorender.com. We thank the patients and clinical teams involved in each of the referenced clinical trials for making this review possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Intramural Research Program of the Center for Cancer Research, NCI, NIH (ZIA BC 010945).
Conflict of interest
N.T. and J.G. are employees of the National Cancer Institute, National Institutes of Health. J.G. is a senior investigator on clinical studies using bintrafusp alfa. The National Cancer Institute has a cooperative research and development agreement with EMD Serono. N.T. and J.G. have no other conflicts of interest to declare that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authorship contributions
N.T. and J.G. conceived of the review scope, drafted the manuscript, and reviewed and approved the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tschernia, N.P., Gulley, J.L. Tumor in the Crossfire: Inhibiting TGF-β to Enhance Cancer Immunotherapy. BioDrugs 36, 153–180 (2022). https://doi.org/10.1007/s40259-022-00521-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00521-1