Abstract
Background
Dexmedetomidine is currently off-label for use in pediatric clinical care worldwide. Nevertheless, it is frequently prescribed to pediatric patients as premedication prior to induction of anesthesia or for procedural sedation. There is ample literature on the pharmacokinetics, efficacy and safety of dexmedetomidine in this vulnerable patient population, but there is a general lack of consensus on dosing. In this project, we aimed to use the standardized workflow of the Dutch Pediatric Formulary to establish best evidence-based pediatric dosing guidelines for dexmedetomidine as premedication and for procedural sedation.
Method
The available literature on dexmedetomidine in pediatrics was reviewed in order to address the following three questions: (1) What is the right dose? (2) What is known about efficacy? (3) What is known about safety? Relevant literature was compiled into a risk–benefit analysis document. A team of clinical experts critically appraised the analysis and the proposed dosing recommendations.
Results
Dexmedetomidine is most commonly administered via the intravenous or intranasal route. Clearance is age dependent, warranting higher doses in infants to reach similar exposure as in adults. Dexmedetomidine use results in satisfactory sedation at parent separation, adequate sedation and a favorable recovery profile. The safety profile is good and comparable to adults, with dose-related hemodynamic effects.
Conclusion
Following the structured approach of the Dutch Pediatric Formulary, best evidence-based dosing recommendations were proposed for dexmedetomidine, used as premedication prior to induction of anesthesia (intranasal dose) and for procedural sedation (intranasal and intravenous dose) in pediatric patients.
Similar content being viewed by others
Dexmedetomidine is frequently used for sedation and analgesia in pediatric patients worldwide, despite the lack of approved pediatric labeling and consensus on dosing. |
The Dutch Pediatric Formulary aims to close the gap between scientific research output and the implementation of dosing recommendations in clinical practice. |
With ample literature on pharmacokinetics, efficacy and safety of dexmedetomidine in the pediatric population, we were able to establish best evidence-based dosing recommendations for dexmedetomidine in pediatric clinical care. |
1 Introduction
Despite the fact that dexmedetomidine is not licensed for use in pediatrics, its use in pediatric clinical care is rapidly increasing [1]. Dexmedetomidine is a highly selective α2-adrenoceptor agonist providing sedation, anxiolysis and sympatholysis. Also, the drug has an analgesic-sparing effect [2]. Compared with other more traditional sedative agents such as barbiturates, midazolam and propofol, dexmedetomidine use is associated with minimal depression of respiratory function [3,4,5]. Several animal studies show evidence of dexmedetomidine being neuroprotective, which is important as neurotoxicity remains a significant concern because of children’s ongoing brain development. Also, there is a growing concern of the possible toxicity of anesthetics in particular as they may induce apoptosis and interfere with neurogenesis. Dexmedetomidine may alleviate brain damage induced by anesthetics [6, 7].
In a recent, large international survey, 70% of nearly 800 anesthesiologists from four international societies who responded to the survey use dexmedetomidine in pediatric clinical practice. The drug is most commonly prescribed for procedural sedation of non-intubated patients (68%), premedication (46%) and/or ICU sedation (46%). The other 30% of the respondents stated that the absence of a protocol and a lack of consensus for dosing were the main reasons for not prescribing dexmedetomidine. This, together with the observation that there is a large variability in the doses being prescribed by physicians, highlights the need for best evidence-based, easily accessible dosing recommendations [8].
To aid decision making for pediatric drug dosing, especially for off-label use, the Dutch Pediatric Formulary (DPF) was developed. This web-based formulary provides pediatric dosing guidelines for all drugs used in children in the Netherlands (approximately 800 drugs, both on-label and off-label) and is the official dosing guideline for pediatrics in the Netherlands. The dosing recommendations are established based on careful selection and appraisal of relevant registration data, investigator-initiated research, professional guidelines and expert opinion [9]. The creation or revision of a drug dosing recommendation is initiated based on medical need as expressed by healthcare professionals. For dexmedetomidine, the DPF received several requests over the last year to establish a uniform dosing recommendation for dexmedetomidine use as premedication and/or for mild sedation during short painful procedures (in combination with local analgesia) or during medical imaging procedures.
In this project, we aimed to establish a best evidence-based dosing recommendation for dexmedetomidine for use as premedication or procedural sedation in pediatric clinical care, using the stepwise approach of the DPF.
2 Methods
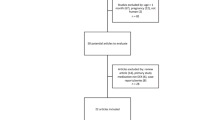
The DPF aims to close the gap between scientific research output and the implementation of best evidence-based dosing recommendations in clinical practice [9]. The stepwise approach to establish dosing recommendations is visualized in Fig. 1.
The formulary uses a standard operating procedure (SOP) to search, select, appraise and summarize available literature to compile a dosing recommendation, with the following three questions in mind:
-
1) What is the right dose from a pharmacokinetic (PK) perspective?
Review of PK parameters is essential to elucidate the relationship between the dose and published target concentrations.
-
2) What is known about efficacy (benefit) and how does it relate to PK properties (the right dose)?
Original literature on the efficacy of the use of the drug in the intended population is reviewed.
-
3) What is known about safety (risk) and how does it relate to PK properties (the right dose)?
Literature is reviewed to identify potential harm of the drug. The relationship between dose and adverse effects is appraised.
The following data sources were used: (i) Registration documents were reviewed to extract information on the registration status and corresponding dosing recommendations, PK and safety (e.g. contraindications, warnings and precautions). (ii) The PubMed database was used to carefully screen scientific literature and extract information on PK, efficacy and safety in pediatrics. The search query can be found in the electronic supplementary material (ESM_1). All relevant available literature was summarized in a risk-benefit analysis (RBA) document, which can be found in ESM_2. In addition to systematic reviews and meta-analyses, which provided valuable information about the overall effect of dexmedetomidine for its use as premedication and/or procedural sedation, original studies were carefully examined and included in the RBA. The evidence level of all relevant individual studies was graded according to their quality (ESM_3). A different grading system was applied for studies on pharmacokinetics versus studies on effect. Both systems encompass five categories. A dose recommendation was synthesized based on available evidence. The RBA document and the proposed dose recommendation were sent to an international multidisciplinary expert team of pharmacists, pediatricians, clinical pharmacologists, medical specialists, etc., and complemented with clinical experience. All team members critically appraised the RBA. Questions or comments and remaining uncertainties were discussed during a multidisciplinary meeting. The meeting was held online, so all international team members could attend and provide input. The RBA document was complemented with comments from the meeting and the definitive RBA document with the final dosing recommendations was distributed among the members of the expert team.
3 Results
Establishing a best evidence-based pediatric dosing recommendation for dexmedetomidine requires a careful assessment of available scientific literature. An RBA was performed following a stepwise approach.
3.1 What is The Right Dose?
3.1.1 General Considerations
3.1.1.1 Elimination
Hepatic metabolism, mainly N-glucuronidation, plays a major role in dexmedetomidine elimination. Also, N-methylation and oxidation by cytochrome P450 (CYP) enzymes (CYP2A6, CYP1A2, CYP2E1, CYP2D6 and CYP2C19) contribute to dexmedetomidine metabolism. Approximately 95% of dexmedetomidine is excreted as metabolites via urine with an additional 4% being excreted via feces. Less than 1% is excreted unchanged via urine [10].
3.1.1.2 Formulation
Dexmedetomidine is only available as solution for infusion [11]. The registered formulations in Europe do not contain toxic excipients contraindicated in the pediatric population. The dexmedetomidine intravenous (IV) fluid (100 µg/mL) can also be used, off-label, for intranasal administration [12]. In case of intranasal administration, a large left-over volume in the atomizer may result in underdosing, so larger volumes may be needed. This may be dependent on the kind of vaporizer syringe that is used.
3.1.2 Adults
The mean estimate of the terminal elimination half-life (t½) is approximately 1.9–2.5 h (min 1.35, max 3.68 h) and the mean estimate of the volume of distribution (Vd) at steady state is approximately 1.16–2.16 L/kg. Plasma clearance (CL) has an estimated value of 0.46–0.73 L/h/kg [11].
The US Food and Drug Administration (FDA) and the European Medicines Agency have licensed dexmedetomidine for use in adults [13, 14]. For adult procedural sedation (sedation of non-intubated patients prior to and/or during diagnostic or surgical procedures requiring sedation, i.e. procedural), a loading infusion of 1 µg/kg is given over 10 min followed by a maintenance infusion of 0.2–1.0 µg/kg/h. The maximum dose of 1.4 µg/kg/h should not be exceeded [11]. The target plasma concentration depends on the required level of sedation, ranging from 200 to 1250 pg/mL. Most frequently, the target plasma concentration is 300–600 pg/mL [10, 14,15,16].
3.1.3 Pediatrics
Knowledge on age-dependent dexmedetomidine PK is of eminent value when carefully establishing a dosing recommendation for specific pediatric age groups. Literature review revealed that PK studies do not necessarily restrict inclusion of subjects to one single indication for which dexmedetomidine could be prescribed. For instance, pediatric patients undergoing urologic surgery, low-risk cardiac surgery, plastic surgery, craniofacial surgery, bronchoscopy, nuclear magnetic resonance imaging or cardiac surgery were all included in the pooled analysis of Potts and colleagues [17]. Studies investigating the PK of dexmedetomidine for indications other than premedication and/or procedural sedation are also taken into consideration as this knowledge is of value for carefully establishing adequate age-appropriate dosing recommendations.
3.1.3.1 Intravenous Administration—Pharmacokinetic Information
We identified a population PK (PopPK) analysis of four PK studies as part of an FDA submission file [18]. Despite the fact that the company performed pediatric trials, the FDA did not grant a pediatric license, as the evidence to support efficacy and safety in pediatrics was deemed insufficient mainly due to issues with study design and patient recruitment. We decided to include the PK data in our evaluation, as these are qualitatively sound.
Table 1 shows the reported PK parameter estimates as geometric mean with 95% confidence interval (CI), by age group. Children received an IV loading dose of 0.05–1 µg/kg and a maintenance infusion of 0.05–2 µg/kg/h postoperatively after cardiac surgery or for sedation in the intensive care unit (ICU) [18].
Weight-adjusted CL values, stratified for age, are somewhat higher than reported by the study of Potts et al. [17], in which PK data of four studies were combined. The PopPK analysis of the FDA included more patients than Potts et al. (n = 131 vs n = 95, respectively) and fewer postcardiac surgery patients. Both simulation studies indicate immature clearance in neonates and higher bodyweight-corrected clearance in infants compared with older children and adults. A lower clearance in neonates is also supported by findings of Chrysostomou et al., Su et al. and Greenberg et al. [13, 19, 20]. Interestingly, van Dijkman et al., using extrapolated doses from the Potts study, found approximately 25% higher clearance in neonates and thereby underexposure. The number of patients is, however, low. Covariate analysis to better understand this variability was not possible yet [21]. Therefore, without adjusting the dose for neonates, a higher steady-state plasma concentration (Css) can be expected, and the opposite for infants [17, 18].
The FDA submission file (for pediatric registration) also included a simulation study of dose-normalized Css upon 1 µg/kg IV loading dose (over 10 min followed by a maintenance infusion of 0.7 µg/kg/h [18]. This dose corresponds to the dose recommended in the label for use in adults. For this analysis, two PK studies were used. The study of Su et al. investigated PK of children (n = 36) after cardiac surgery [13]. To the best of our knowledge, the study results of the second PK study involving children receiving dexmedetomidine for sedation in the ICU (n = 58) were not published in a peer-reviewed journal. The results of this simulation study are shown in Table 2.
Interestingly, the resulting Css is relatively high compared with the most frequently used target plasma concentration in adults (generally 300–600 pg/mL). It is important to note that a target plasma concentration for pediatrics is not formally defined and it is assumed that the optimal target range in pediatrics is similar to that in adults, but obviously depends on the level of sedation that is required [17].
A simulation study by Potts et al. suggested that plasma concentrations between 400 and 800 pg/mL are required to achieve mild to moderate sedation in children, which roughly aligns with the recommended target in adults. To achieve the target concentration of 600 pg/mL, age- and indication-specific dosing recommendations were suggested [17] (Table 3).
3.1.3.2 Intravenous Administration—Procedural Sedation
Loading dose A systematic review and meta-analysis by Rao et al. showed that loading doses ranging from 0.5 to 2.5 µg/kg in 10 minutes have been applied in a total of 10 studies (with varying indications) in children, and the model by Potts et al. suggests using a loading dose of 0.6 µg/kg in 10 minutes to quickly reach steady state concentrations sufficient to achieve mild to moderate sedation [17, 22]. No association between dexmedetomidine exposure and adverse events was found with the use of small loading doses of 0.1–3 µg/kg, followed by maintenance doses of 0.1–2.5 µg/kg/h [20]. If a loading dose is not used, onset of sedation may be extended, due to the relatively long dexmedetomidine elimination half-life. Based on PK data, a loading dose for procedural sedation of 0.5–1 µg/kg in 10 minutes seems appropriate.
Maintenance dose A higher bodyweight-corrected CL was found in children compared with adults. Taking into consideration all studies included in the study Rao et al., a maintenance dose between 0.1 and 2 µg/kg/h is applied, with most studies using dose of, or close to, 0.5 µg/kg/h [22]. Therefore, based on PK data combined with target concentrations associated with sedation, as well as safety data, we propose a maintenance dose of 0.1–1.4 µg/kg/h for children aged ≥1 month for procedural sedation. The dose depends on the required level of sedation and can be titrated based on effect up to a maximum dose of 2.5 µg/kg/h, if hemodynamically tolerated.
3.1.3.3 Intranasal Administration—Premedication and Procedural Sedation
For premedication or when an IV access is not in place yet, intranasal (IN) administration is the preferable route. Dexmedetomidine IN may be preferred over IV use to avoid pain and distress caused by IV cannulation or in case obtaining IV access is difficult. To study PK upon intranasal administration, 18 children aged 6–44 months received a single intranasal dose after elective cardiac surgery. Atomized intranasal administration of 1 µg/kg resulted in a plasma concentration of approximately 100 pg/mL (low end reported for sedative efficacy) within 20 minutes (peak: 199 pg/mL in 46 min) while this concentration is reached within 10 minutes with a dose of 2 µg/kg (also, approx. 2 × higher maximum concentration [Cmax]). The typical bioavailability, simulated with PopPK modelling, was 83.8% (95% CI 69.5–98.1). The Cmax and time to Cmax (tmax) (median with range) found are shown in Table 4 [23].
A meta-analysis of 13 randomized controlled trials on the use of dexmedetomidine as premedication prior to induction of anesthesia showed that a wide range of IN doses are being used, from 0.5 to 4 µg/kg [24]. Three other recently published large systematic reviews on the use of dexmedetomidine for procedural sedation or premedication showed us that a single IN dose of 1 µg/kg is most commonly used and we therefore recommend this dose to be used for premedication or procedural sedation [25,26,27].
3.1.3.4 Cardiac Surgery
Children who underwent cardiac surgery may have a lower dexmedetomidine CL immediately postoperatively. This observation can be explained by a reduced cardiac output following cardiac surgery. Reduced clearance can be attributed to hepatic hypoperfusion. The effect is transient however, as hepatic perfusion increases again during the first few days post-surgery [17, 20].
3.2 What is Known About Efficacy?
3.2.1 Indication: Premedication (Prior to Induction of Anesthesia)
3.2.1.1 Intranasal Use
Between 2009 and 2020, 49 systematic reviews/meta-analyses were published on dexmedetomidine in pediatrics of which five focused on the use of dexmedetomidine as premedication [24,25,26, 28, 29]. Because of substantial overlap of included studies in these systematic reviews/meta-analyses, the three most recently published reviews of Jun et al., Feng et al. and Pasin et al. were used for the RBA [24,25,26]. All three reviews assessed the quality of the included randomized controlled trials by assessing the risk of selection bias, performance bias, detection bias and reporting bias using the Cochrane risk of bias tool. A potential risk for selection bias was most frequently reported, but overall the risk of bias was considered to be very low. While Feng et al. and Pasin et al. only performed statistical analyses on the outcome (reporting risk ratios with 95% confidence intervals and I2 test for heterogeneity), Jun et al. also assessed the quality of the evidence for the effect of dexmedetomidine on the outcomes of interest. However, the GRADE approach showed that the overall quality of evidence was low or moderate and that inconsistency and imprecision play a major role [24,25,26]. Statistical analyses of the studies showed that dexmedetomidine use is associated with satisfactory sedation at parent separation and that there is less need for postoperative rescue analgesics upon preanesthetic use of dexmedetomidine. All three studies showed that these effects were statistically significant (p < 0.05). Most commonly, dexmedetomidine was given IN, with doses ranging from 0.5 to 2 µg/kg and given 30–60 minutes before surgery. A single IN dose of 1 µg/kg is most frequently used. Based on efficacy studies, an IN dose range of 1–2 µg/kg seems rational for dexmedetomidine use as premedication.
3.2.2 Indication: Procedural Sedation of Non-Intubated Patients
3.2.2.1 Intranasal and Intravenous Use
The majority of the 49 systematic reviews/meta-analyses focused on the pediatric use of dexmedetomidine for procedural sedation and its frequent use in the prevention of postoperative side effects. Based on the publication date and the number of included studies, the systematic reviews/meta-analysis of Lian et al. and Rao et al. were selected to assess the efficacy of dexmedetomidine for procedural sedation and evaluate the effect on postoperative recovery. Rao et al. focused on the prevention of emergence agitation and delirium primarily, and Lian et al. evaluated several outcome parameters related to the success of sedation [22, 27]. Of the 58 randomized controlled trials included by Rao et al., 19 trials were assessed to be of high quality as the risk for selection bias, performance bias, attrition bias and reporting bias was found to be low. For most of the moderate- to low-quality studies, there was a risk for selection and performance bias. The meta-analysis showed that dexmedetomidine effectively reduces the incidence of emergence agitation and delirium (p < 0.05). Interestingly, the route of administration differs between studies in Rao et al. (IV: n = 39, IN: n = 12, other: n = 12). Various dosing strategies are being used for IV dosing: a single IV dose is being used in 20 trials, a loading dose plus maintenance infusion in 10 trials and only maintenance infusion in 6 trials. An overview of high-quality studies included by Rao et al. is given in the RBA document (ESM_2) [22]. In case a single IV or IN dose is given to prevent emergence delirium, strict timing is not deemed to be necessary because of the relatively long duration of action [30]. Lian et al. reported a high success rate of sedation with dexmedetomidine. Also, dexmedetomidine is effective in reducing the incidence of postoperative vomiting, crying and/or resisting. In general, pediatric dexmedetomidine use is associated with adequate sedation and it has a favorable postoperative recovery. It should, however, be noted that 9 out of 15 studies included in this systemic review/meta-analysis scored relatively highly with respect to the risk of bias (≥4 of the 7 aspects of bias scored as unknown or high risk) [27]. Based on efficacy studies, an IN dose of 2–3 µg/kg and an IV loading dose of 0.5–2 µg/kg in 10 minutes followed by a maintenance dose of 0.5–1.5 µg/kg/h seems rational for procedural sedation.
3.3 What is Known About Safety?
3.3.1 General Information
In general, the use of dexmedetomidine in children appears to be safe. In a study by Greenberg et al., with 20 children with a postmenstrual age ranging between 33 and 61 weeks, no association between dexmedetomidine exposure and adverse events was found [20]. Despite the fact that safety issues may not be of major concern in the case of short-term dexmedetomidine use as premedication or for procedural sedation, a description of the potential risks is essential to make well-informed dosing decisions. Adverse events are, generally, not indication specific.
3.3.2 Cardiovascular Effects
Dexmedetomidine is an α2-agonist and side effects are mainly cardiovascular. Many studies report no adverse events, a few studies have reported cases of hypotension and bradycardia leading to discontinuation of dexmedetomidine and in some cases requiring resuscitation measures [13, 19, 31]. Hauber et al. reported that the hemodynamic effects of dexmedetomidine are related to the rate of infusion and to the dose used—bradycardia and a biphasic arterial blood pressure response characterized by a transient initial increase followed by a decrease upon dexmedetomidine administration [32]. This effect was similar to what has been observed in adults. While one study shows that premedication with atropine could be used to prevent dexmedetomidine-induced bradycardia, it should be noted that the adverse effects of anti-cholinergic treatment, characterized by a considerable increase in systolic blood pressure, is likely to outweigh the potential benefits (preventing bradycardia) [33, 34]. The incidence and risks of bradycardia in dexmedetomidine-treated children is small and no cases of pharmacologic treatment have been reported [6]. In addition to bradycardia, a study in male healthy volunteers showed a biphasic response of the mean arterial pressure (MAP) on dexmedetomidine plasma levels, with a high plasma concentration resulting in an increased MAP and a lower plasma concentration resulting in a decreased MAP [11, 35]. It is, however, important to note that despite these effects, hemodynamic collapse and/or the need for pharmacologic intervention have not been described [6, 36].
3.3.3 Contraindications and Warnings
The label states that the use of dexmedetomidine in adults with advanced heart block (grade 2 or 3) is contraindicated, unless patients have a pacemaker. Also, patients with uncontrolled hypotension or acute cerebrovascular conditions should not use dexmedetomidine. In addition, cardiac monitoring of patients using dexmedetomidine is essential. Despite the minimal risk of respiratory depression and apnea, respiratory function should be monitored, also in non-intubated patients [11].
Lin and Ansermino reported a list of conditions warranting precaution when using dexmedetomidine in children [36], comprising (i) the use of rapid bolus of dexmedetomidine with high blood concentrations of volatile anesthetics, (ii) cardiac conduction abnormalities, (iii) symptoms of septic shock or diagnosed septic shock, (iv) use of digoxin, β-adrenergic blockers, calcium channel blockers, monoamine oxidase (MAO) inhibitors or other agents that predispose to bradycardia or hypotension as comedication, (v) current hypertension and (vi) hepatic abnormality.
3.4 Review By the Expert Team
The PK, efficacy and safety of dexmedetomidine has been explored in many studies, where it was used as premedication, for procedural analgosedation and/or the prevention of post-operative emergence of delirium, nausea and vomiting. Members of the expert team confirmed that dexmedetomidine is used as an add-on agent to other drugs inducing anesthesia to mitigate any negative effects of these drugs. Dexmedetomidine is not used as an analgesic drug during surgical procedures in an emergency setting. Indications for use were defined as premedication prior to induction of anesthesia and procedural sedation. With conflicting dosing strategies reported in the literature, we aim to provide harmonized best evidence-based dosing recommendation for these indications.
First, IN dosing of dexmedetomidine was discussed. Dexmedetomidine can be administered intranasally prior to induction of anesthesia, as premedication or can be used for procedural sedation. A deeper level of sedation is required when dexmedetomidine is used during certain procedures as compared with its use as premedication. Too little information from scientific literature is available to provide a specific dose for a particular level of sedation. Therefore, an IN dose of 1–2 µg/kg is recommended when dexmedetomidine is used as premedication and 2–3 µg/kg dexmedetomidine is proposed for procedural sedation. With respect to the latter, when dexmedetomidine is used in pain management as a co-analgesic, lower doses are needed, while doses at the upper range are more appropriate when the drug is used for sedation (based on expert opinion). Norwegian experts indicate that higher doses, up to 4 µg/kg, are being used, but this is not widely supported by literature. The expert team decided to exclude children <6 months of age for the IN dose recommendations for both indications because of (i) the lack of a good indication as neonates are rarely sedated with dexmedetomidine outside the ICU setting, (ii) the lack of safety and efficacy data in neonates and (iii) practical issues (low volumes to administer).
Secondly, the expert team discussed IV dosing of dexmedetomidine for procedural sedation. While some studies and experts suggest that only a single IV dose can be used, others indicate that a loading dose must be followed by a maintenance infusion. Also, some indicate that only a continuous infusion is appropriate. Clinical experts clarify that the decision to use a loading dose and/or maintenance dose depends on the expected severity of post-operative pain and the duration of the procedure. It is not a one-size-fits-all approach. Considering the relatively long half-life of the drug, a loading dose is rational to attain timely steady-state concentration in prolonged sedation. To mitigate the hemodynamic effects of a loading dose, it is suggested to follow the 10-min infusion time for a loading dose according to the label for adults. Based on PK and efficacy data, a loading dose of 0.5–0.1 µg/kg in 10 min followed by a maintenance dose of 0.1–1.4 µg/kg/h (max. 2.5 µg/kg/h) was proposed. Taking into consideration the evidence reported by Rao et al., with loading doses ranging from 0.15 to 2.5 µg/kg and maintenance doses of 0.1–2 µg/kg/h [22], the expert team agreed upon a loading dose of 0.5–2 µg/kg in 10 min followed by a maintenance dose of 0.5–1.5 µg/kg/h. In addition, the expert team decided to include a single IV dose recommendation of 0.5–1 µg/kg for the prevention of postoperative agitation and/or delirium, based on the studies of Hauber et al. (0.3–1 µg/kg), Tsiotou et al. (≥ 0.5 µg/kg) and Song et al. (1 µg/kg) [32, 37, 38].
At this stage, it was decided not to include a dosing recommendation for neonates. Outside the operating room and ICU, neonates are rarely sedated for a minor procedure due to a higher risk for complications. If, in the near future, a dosing recommendation for sedation in the ICU is established, neonates can be included. It is important to note that neonates are highly susceptible to the cardiac effects of dexmedetomidine and therefore its use can only be recommended in this vulnerable patient population if continuous cardiac monitoring can be guaranteed and if facilities for cardiac resuscitation are available. With respect to IN dosing of dexmedetomidine, Bua et al. describe the favorable safety and efficacy profile of dexmedetomidine in neonates undergoing MRI [39]. The expert team, however, indicated that the lack of an adequate age-appropriate formulation and uncertainty about the dose delivered by the atomizer device hinders the inclusion of this age group in the dosing guideline that is yet to be established. This does not only hold true for neonates, but for pediatric patients below the age of 6 months. For this reason, and supported by the meta-analyses of Li et al., it was decided to exclude intranasal use for neonates and infants younger than 6 months of age [40].
3.5 Identified Risks and Benefits
3.5.1 Benefits
Compared with other more traditional sedative agents, dexmedetomidine use is associated with minimal depression of respiratory function as it induces a natural state of sleep. Several studies show evidence of dexmedetomidine being neuro-, reno- and cardioprotective [6]. Also, a reduced incidence of dysrhythmias following cardiac surgery has been reported [41, 42]. High-quality studies confirm the efficacy of dexmedetomidine as premedication (i.e. satisfactory sedation at parent separation, reduced need for rescue analgesics, better acceptance of mask induction), for analgosedation during diagnostic or surgical procedures and in mitigating the negative effects of other agents (i.e. prevention of postoperative nausea and vomiting and emergence agitation and/or delirium).
3.5.2 Risks
Hemodynamic side effects are most frequently reported. Administration under close cardiac monitoring (ICU, operating theater) is essential to identify side effects at an early stage. In addition, respiratory function should be monitored in non-ventilated patients. A loading dose is associated with a higher risk of hypertension in adults. There is limited clinical experience with the use of dexmedetomidine in the neonatal population in the Netherlands.
4 Final Dose Recommendation
Based on the review of scientific evidence, we have established an IN dosing recommendation for dexmedetomidine used as premedication in pediatric patients aged between 6 months and 18 years of 1–2 µg/kg once prior to induction of anesthesia. For procedural sedation, an IN dose of 2–3 µg/kg/dose can be used for pediatric patients between 6 months and 18 years of age. The dose can be repeated if needed, with a maximum total dose of 200 µg. Also, an IV dose is now provided for procedural sedation; for patients between 1 month and 18 years of age, a loading dose of 0.5–2 µg/kg can be given over 10 minutes followed by a maintenance dose of 0.5–1.5 µg/kg/h. The dose depends on the required depth of sedation. For the prevention of postoperative agitation and/or delirium, a single IV dose of 0.5–1 µg/kg can be given before the end of anesthesia. An overview of the dosing recommendations is provided in Table 5.
5 Conclusion
Dexmedetomidine is one of the many drugs that is not licensed for use in pediatrics but used on a daily basis in clinical practice [43]. Despite the fact that there is a substantial body of evidence on dexmedetomidine use in pediatrics, including almost 50 systematic reviews and meta-analyses (last searched January 2021), studies focusing on finding and explicitly stating the most optimal doses in different pediatric age groups are scarce. Frequently, physicians prescribing the drug to the patient are not in a position to review all available literature and stay up to date. It is clear that there is an urgent demand to bridge the gap between sound scientific evidence and clinical practice. The work described herein represents an important step towards implementation of evidence-based dosing recommendations into clinical practice. Recently, the BRAvO (Benefit and Risk Assessment for Off-label use) framework was published describing whether and how to perform a benefit–risk analysis for off-label pediatric prescribing [44]. This framework, encompassing eight critical elements, will pave the way towards a standardized and thorough assessment of benefits and risks to ultimately ensure safe and effective pharmacotherapy in pediatrics.
From our example, it can be learned that establishing dosing advice for a specific drug requires a tailored approach and a solid and structured framework; template documents and SOPs are essential in order to compile all relevant information and to present the findings in an efficient and transparent manner to a team of experts.
References
DHHS and FDA. Pediatric postmarketing pharmacovigilance and drug utilization review. Dexmedetomidine.
Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. https://doi.org/10.1007/s40262-017-0507-7.
Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103(1):63–7. https://doi.org/10.1213/01.ANE.0000219592.82598.AA.
Oshima H, Nakamura M, Watanabe O, Yamamura T, Funasaka K, Ohno E, et al. Dexmedetomidine provides less body motion and respiratory depression during sedation in double-balloon enteroscopy than midazolam. SAGE Open Med. 2017;5:2050312117729920. https://doi.org/10.1177/2050312117729920.
Tobias JD, Leder M. Procedural sedation: a review of sedative agents, monitoring, and management of complications. Saudi J Anaesth. 2011;5(4):395–410. https://doi.org/10.4103/1658-354X.87270.
Mahmoud M, Barbi E, Mason KP. Dexmedetomidine: what’s new for pediatrics? A narrative review. J Clin Med. 2020. https://doi.org/10.3390/jcm9092724.
Sinner B, Becke K, Engelhard K. General anaesthetics and the developing brain: an overview. Anaesthesia. 2014;69(9):1009–22. https://doi.org/10.1111/anae.12637.
van Hoorn CE, Flint RB, Skowno J, Davies P, Engelhardt T, Lalwani K, et al. Off-label use of dexmedetomidine in paediatric anaesthesiology: an international survey of 791 (paediatric) anaesthesiologists. Eur J Clin Pharmacol. 2021;77(4):625–35. https://doi.org/10.1007/s00228-020-03028-2.
van der Zanden TM, de Wildt SN, Liem Y, Offringa M, de Hoog M. Dutch paediatric pharmacotherapy expertise network N. Developing a paediatric drug formulary for the Netherlands. Arch Dis Child. 2017;102(4):357–61. https://doi.org/10.1136/archdischild-2016-311674.
EMA. Assessment report. Dexdor. Dexmedetomidine. https://www.ema.europa.eu/en/documents/assessment-report/dexdor-epar-public-assessment-report_en.pdf. Accessed Jan 2021
EMA. Summary of product characteristics. Dexmedetomidine Accord. https://www.ema.europa.eu/en/documents/product-information/dexmedetomidine-accord-epar-product-information_en.pdf. Accessed Jan 2021
Wang L, Huang L, Zhang T, Peng W. Comparison of Intranasal Dexmedetomidine and Oral Midazolam for Premedication in Pediatric Dental Patients under General Anesthesia: A Randomised Clinical Trial. Biomed Res Int. 2020;2020:5142913. https://doi.org/10.1155/2020/5142913.
Su F, Gastonguay MR, Nicolson SC, DiLiberto M, Ocampo-Pelland A, Zuppa AF. Dexmedetomidine pharmacology in neonates and infants after open heart surgery. Anesth Analg. 2016;122(5):1556–66. https://doi.org/10.1213/ANE.0000000000000869.
FDA. Highlights of Prescribing Information. Predecex. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206628s000lbl.pdf. Accessed Jan 2021.
Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 1: Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83(3):372–80. https://doi.org/10.1093/bja/83.3.372.
Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101(5):1066–76. https://doi.org/10.1097/00000542-200411000-00005.
Potts AL, Anderson BJ, Warman GR, Lerman J, Diaz SM, Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care—a pooled analysis. Paediatr Anaesth. 2009;19(11):1119–29. https://doi.org/10.1111/j.1460-9592.2009.03133.x.
FDA-Clinical_Pharmacology_Review. Pediatric Submission. 12/17/2012. Accessed Jan 2021. https://www.fda.gov/media/86351/download.
Chrysostomou C, Schulman SR, Herrera Castellanos M, Cofer BE, Mitra S, da Rocha MG, et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164(2):276–82. https://doi.org/10.1016/j.jpeds.2013.10.002.
Greenberg RG, Wu H, Laughon M, Capparelli E, Rowe S, Zimmerman KO, et al. Population pharmacokinetics of dexmedetomidine in infants. J Clin Pharmacol. 2017;57(9):1174–82. https://doi.org/10.1002/jcph.904.
van Dijkman SC, De Cock P, Smets K, Decaluwe W, Smits A, Allegaert K, et al. Dose rationale and pharmacokinetics of dexmedetomidine in mechanically ventilated new-borns: impact of design optimisation. Eur J Clin Pharmacol. 2019;75(10):1393–404. https://doi.org/10.1007/s00228-019-02708-y.
Rao Y, Zeng R, Jiang X, Li J, Wang X. The effect of dexmedetomidine on emergence agitation or delirium in children after anesthesia-a systematic review and meta-analysis of clinical studies. Front Pediatr. 2020;8:329. https://doi.org/10.3389/fped.2020.00329.
Miller JW, Balyan R, Dong M, Mahmoud M, Lam JE, Pratap JN, et al. Does intranasal dexmedetomidine provide adequate plasma concentrations for sedation in children: a pharmacokinetic study. Br J Anaesth. 2018;120(5):1056–65. https://doi.org/10.1016/j.bja.2018.01.035.
Pasin L, Febres D, Testa V, Frati E, Borghi G, Landoni G, et al. Dexmedetomidine vs midazolam as preanesthetic medication in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2015;25(5):468–76. https://doi.org/10.1111/pan.12587.
Feng JF, Wang XX, Lu YY, Pang DG, Peng W, Mo JL. Effects of dexmedetomidine versus midazolam for premedication in paediatric anaesthesia with sevoflurane: a meta-analysis. J Int Med Res. 2017;45(3):912–23. https://doi.org/10.1177/0300060517704595.
Jun JH, Kim KN, Kim JY, Song SM. The effects of intranasal dexmedetomidine premedication in children: a systematic review and meta-analysis. Can J Anaesth. 2017;64(9):947–61. https://doi.org/10.1007/s12630-017-0917-x.
Lian X, Lin Y, Luo T, Yuan H, Chen Y. Comparison of dexmedetomidine with chloral hydrate as sedatives for pediatric patients: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(31): e21008. https://doi.org/10.1097/MD.0000000000021008.
Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: a systematic review and meta-analysis. Clinics (Sao Paulo). 2014;69(11):777–86. https://doi.org/10.6061/clinics/2014(11)12.
Sun Y, Lu Y, Huang Y, Jiang H. Is dexmedetomidine superior to midazolam as a premedication in children? A meta-analysis of randomized controlled trials. Paediatr Anaesth. 2014;24(8):863–74. https://doi.org/10.1111/pan.12391.
Cho EA, Cha YB, Shim JG, Ahn JH, Lee SH, Ryu KH. Comparison of single minimum dose administration of dexmedetomidine and midazolam for prevention of emergence delirium in children: a randomized controlled trial. J Anesth. 2020;34(1):59–65. https://doi.org/10.1007/s00540-019-02705-6.
Pan W, Wang Y, Lin L, Zhou G, Hua X, Mo L. Outcomes of dexmedetomidine treatment in pediatric patients undergoing congenital heart disease surgery: a meta-analysis. Paediatr Anaesth. 2016;26(3):239–48. https://doi.org/10.1111/pan.12820.
Hauber JA, Davis PJ, Bendel LP, Martyn SV, McCarthy DL, Evans MC, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg. 2015;121(5):1308–15. https://doi.org/10.1213/ANE.0000000000000931.
Ahn EJ, Park JH, Kim HJ, Kim KW, Choi HR, Bang SR. Anticholinergic premedication to prevent bradycardia in combined spinal anesthesia and dexmedetomidine sedation: a randomized, double-blind, placebo-controlled study. J Clin Anesth. 2016;35:13–9. https://doi.org/10.1016/j.jclinane.2016.07.012.
Subramanyam R, Cudilo EM, Hossain MM, McAuliffe J, Wu J, Patino M, et al. To pretreat or not to pretreat: prophylactic anticholinergic administration before dexmedetomidine in pediatric imaging. Anesth Analg. 2015;121(2):479–85. https://doi.org/10.1213/ANE.0000000000000765.
Josephine C, Shariffuddin II, Chaw SH, Ng KWS, Ng KT. Hemodynamic response of high- and low-dose dexmedetomidine of pediatric in general anesthesia: a systematic review and meta-analysis of randomized controlled trials. Asian J Anesthesiol. 2021. https://doi.org/10.6859/aja.202101/PP.0003.
Lin R, Ansermino JM. Dexmedetomidine in paediatric anaesthesia. BJA Educ. 2020;20(10):348–53. https://doi.org/10.1016/j.bjae.2020.05.004.
Tsiotou AG, Malisiova A, Kouptsova E, Mavri M, Anagnostopoulou M, Kalliardou E. Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: a double-blind, randomized study. Paediatr Anaesth. 2018;28(7):632–8. https://doi.org/10.1111/pan.13397.
Song IA, Seo KS, Oh AY, Baik JS, Kim JH, Hwang JW, et al. Dexmedetomidine injection during strabismus surgery reduces emergence agitation without increasing the oculocardiac reflex in children: a randomized controlled trial. PLoS ONE. 2016;11(9):e0162785. https://doi.org/10.1371/journal.pone.0162785.
Bua J, Massaro M, Cossovel F, Monasta L, Brovedani P, Cozzi G, et al. Intranasal dexmedetomidine, as midazolam-sparing drug, for MRI in preterm neonates. Paediatr Anaesth. 2018;28(8):747–8. https://doi.org/10.1111/pan.13454.
Li L, Zhou J, Yu D, Hao X, Xie Y, Zhu T. Intranasal dexmedetomidine versus oral chloral hydrate for diagnostic procedures sedation in infants and toddlers: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(9): e19001. https://doi.org/10.1097/MD.0000000000019001.
Tobias JD, Chrysostomou C. Dexmedetomidine: antiarrhythmic effects in the pediatric cardiac patient. Pediatr Cardiol. 2013;34(4):779–85. https://doi.org/10.1007/s00246-013-0659-7.
Wang W, Liu J, Ye H, Wang M, Wang T. Effect of dexmedetomidine on tachyarrhythmias after cardiac surgery: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2021. https://doi.org/10.1097/FJC.0000000000001196.
Report: Study on off-label use of medicinal products in the European Union. NIVEL. European Union, 2017
van der Zanden TM, Mooij MG, Vet NJ, Neubert A, Rascher W, Lagler FB, et al. Benefit-risk assessment of off-label drug use in children: the bravo framework. Clin Pharmacol Ther. 2021;110(4):952–65. https://doi.org/10.1002/cpt.2336.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for the research, authorship and/publication of this article.
Conflict of interest
All authors declare that they have no conflict of interest.
Statement of data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Freriksen, J.J.M., van der Zanden, T.M., Holsappel, I.G.A. et al. Best Evidence-Based Dosing Recommendations for Dexmedetomidine for Premedication and Procedural Sedation in Pediatrics: Outcome of a Risk-Benefit Analysis By the Dutch Pediatric Formulary. Pediatr Drugs 24, 247–257 (2022). https://doi.org/10.1007/s40272-022-00498-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00498-y