Abstract
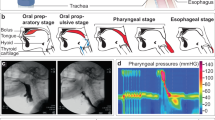
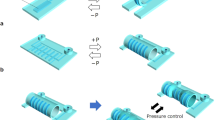
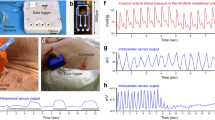
The evaluation of the tone and contractile patterns of the gastrointestinal (GI) tract via manometry is essential for the diagnosis of GI motility disorders. However, manometry is expensive and relies on complex and bulky instrumentation. Here we report the development and performance of an inexpensive and easy-to-manufacture catheter-like device for capturing manometric data across the dynamic range observed in the human GI tract. The device, which we designed to resemble the quipu—knotted strings used by Andean civilizations for the capture and transmission of information—consists of knotted piezoresistive pressure sensors made by infusing a liquid metal (eutectic gallium-indium) through thin silicone tubing. By exploring a range of knotting configurations, we identified optimal design schemes that led to sensing performances comparable to those of commercial devices for GI manometry, as we show for the sensing of GI motility in multiple anatomic sites of the GI tract of anaesthetized pigs. Disposable and customizable piezoresistive catheters may broaden the use of GI manometry in low-resource settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for the figures are available from figshare with the identifiers https://doi.org/10.6084/m9.figshare.14544453 (Fig. 4) and https://doi.org/10.6084/m9.figshare.17434874 (Fig. 5).
References
Fox, M. R. et al. Clinical measurement of gastrointestinal motility and function: who, when and which test? Nat. Rev. Gastroenterol. Hepatol. 15, 568–579 (2018).
Peery, A. F. et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 156, 254–272 (2019).
Anderson, P. et al. Survey of digestive health across Europe: final report. Part 2: the economic impact and burden of digestive disorders. United European Gastroenterol. J. 2, 544–546 (2014).
Moosavi, S., Rezaie, A., Pimentel, M. & Pichetshote, N. Atlas of High-resolution Manometry, Impedance, and pH Monitoring (Springer, 2020).
Saltman, R., Bankauskaite, V. & Vrangbaek, K. Decentralization in Health Care. Strategies and Outcomes (McGraw-Hill, 2007).
Aas, I. M. Organizational decentralization in radiology. J. Telemed. Telecare 12, 1–3 (2006).
Al-Alusi, M. A., Ding, E., McManus, D. D. & Lubitz, S. A. Wearing your heart on your sleeve: the future of cardiac rhythm monitoring. Curr. Cardiol. Rep. 21, 158 (2019).
Isakadze, N. & Martin, S. S. How useful is the smartwatch ECG? Trends Cardiovasc. Med. 30, 442–448 (2020).
Ascher, M. The logical-numerical system of Inca quipus. IEEE Ann. Hist. Comput. 5, 268–278 (1983).
Ascher, M. & Ascher, R. Mathematics of the Incas: Code of the Quipu (Courier Corporation, 2013).
Dickey, M. D. et al. Eutectic gallium‐indium (EGaIn): a liquid metal alloy for the formation of stable structures in microchannels at room temperature. Adv. Funct. Mater. 18, 1097–1104 (2008).
Cooper, C. B. et al. Stretchable capacitive sensors of torsion, strain, and touch using double helix liquid metal fibers. Adv. Funct. Mater. 27, 1605630 (2017).
Yeo, J. C., Yu, J., Koh, Z. M., Wang, Z. & Lim, C. T. Wearable tactile sensor based on flexible microfluidics. Lab Chip 16, 3244–3250 (2016).
Gao, Y. et al. Wearable microfluidic diaphragm pressure sensor for health and tactile touch monitoring. Adv. Mater. 29, 1701985 (2017).
Ma, Z. et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 20, 859–868 (2021).
Holloway, R. H. Esophageal manometry. GI Motility online https://www.nature.com/gimo/contents/pt1/full/gimo30.html (2006).
Liem, O. et al. Solid‐state vs water‐perfused catheters to measure colonic high‐amplitude propagating contractions. Neurogastroenterol. Motil. 24, 345-e167 (2012).
Lu, Y. et al. Transformable liquid-metal nanomedicine. Nat. Commun. 6, 10066 (2015).
Yi, L. & Liu, J. Liquid metal biomaterials: a newly emerging area to tackle modern biomedical challenges. Int. Mater. Rev. 62, 415–440 (2017).
Kim, K. et al. Highly sensitive and wearable liquid metal-based pressure sensor for health monitoring applications: integration of a 3D‐printed microbump array with the microchannel. Adv. Healthc. Mater. 8, 1900978 (2019).
Chen, L. et al. Icing performance of superhydrophobic silicone rubber surfaces by laser texturing. Mater. Res. Express 6, 1250e2 (2020).
Araromi, O. A. et al. Ultra-sensitive and resilient compliant strain gauges for soft machines. Nature 587, 219–224 (2020).
Han, M. et al. Catheter-integrated soft multilayer electronic arrays for multiplexed sensing and actuation during cardiac surgery. Nat. Biomed. Eng. 4, 997–1009 (2020).
Bredenoord, A. J. et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol. Motil. 24, 57–65 (2012).
Hansen, M. B. Small intestinal manometry. Physiol. Res. 51, 541–556 (2002).
Pfeifer, J. & Oliveira, L. in Constipation (eds Wexner, S. D. & Duthie, D. S.) 71–83 (Springer, 2006).
Shaker, A. et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am. J. Gastroenterol. 108, 1706–1712 (2013).
Leber, A. et al. Soft and stretchable liquid metal transmission lines as distributed probes of multimodal deformations. Nat. Electron. 3, 316–326 (2020).
Yu, X. et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 575, 473–479 (2019).
Bellinger, A. M. et al. Oral, ultra-long-lasting drug delivery: application toward malaria elimination goals. Sci. Transl. Med. 8, 365ra157 (2016).
Hosotsubo, M., Magota, T., Egusa, M., Miyawaki, T. & Matsumoto, T. Fabrication of artificial food bolus for evaluation of swallowing. PLoS ONE 11, e0168378 (2016).
Cheeney, G., Nguyen, M., Valestin, J. & Rao, S. S. C. Topographic and manometric characterization of the recto‐anal inhibitory reflex. Neurogastroenterol. Motil. 24, e147–e154 (2012).
Lee, T. H. & Bharucha, A. E. How to perform and interpret a high-resolution anorectal manometry test. J. Neurogastroenterol. Motil. 22, 46–59 (2016).
Gourcerol, G. et al. Do endoflip assessments of anal sphincter distensibility provide more information on patients with fecal incontinence than high‐resolution anal manometry? Neurogastroenterol. Motil. 28, 399–409 (2016).
Zifan, A., Sun, C., Gourcerol, G., Leroi, A. M. & Mittal, R. K. Endoflip vs high‐definition manometry in the assessment of fecal incontinence: a data‐driven unsupervised comparison. Neurogastroenterol. Motil. 30, e13462 (2018).
De Schepper, H. U. et al. Impact of spatial resolution on results of esophageal high‐resolution manometry. Neurogastroenterol. Motil. 26, 922–928 (2014).
Sundaram, S. et al. Learning the signatures of the human grasp using a scalable tactile glove. Nature 569, 698–702 (2019).
Xi, W. et al. Soft tubular microfluidics for 2D and 3D applications. Proc. Natl Acad. Sci. USA 114, 10590–10595 (2017).
Luo, Y. et al. Learning human–environment interactions using conformal tactile textiles. Nat. Electron. 4, 193–201 (2021).
Acknowledgements
We thank A. M. Hayward, K. Ishida and J. Jenkins for supervising and performing the in vivo experiments using Yorkshire swine models; M. Kolle for insightful discussions on knot mechanics; and H. Luan for helping with finite-element modelling. This work was supported in part by the Karl van Tassel (1925) Career Development Professorship and the Department of Mechanical Engineering, MIT.
Author information
Authors and Affiliations
Contributions
K.N. and G.T. conceived and designed the study. K.N. fabricated and characterized the QUILT. S.B., C.M.P. and A.M.J. performed the mechanical analysis and the finite-element modelling. J.L.P.K., K.N., S.S.S. and V.R.F. performed the in vivo experiments on porcine models. V.R.F. synthesized and optimized the artificial food bolus. W.W.C. advised on the clinical aspects of this project and wrote a substantial portion of the manuscript. All authors discussed and interpreted the results, and participated in writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors report a patent application (U.S. Provisional Application No. 63/301,491) describing the system described for manometric evaluation. Complete details of all for-profit and not-for-profit relationships for G.T. are included in the Supplementary Information. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Michael Dickey, Pankaj Pasricha and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1, Figs. 1–19, References, video captions and details of competing interests for G.T.
Supplementary Video 1
Formation of an elastic overhand knot using finite-element simulations.
Supplementary Video 2
Finite-element simulations of knot compression.
Rights and permissions
About this article
Cite this article
Nan, K., Babaee, S., Chan, W.W. et al. Low-cost gastrointestinal manometry via silicone–liquid-metal pressure transducers resembling a quipu. Nat. Biomed. Eng 6, 1092–1104 (2022). https://doi.org/10.1038/s41551-022-00859-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00859-5
This article is cited by
-
In-vivo integration of soft neural probes through high-resolution printing of liquid electronics on the cranium
Nature Communications (2024)
-
Conductive and elastic bottlebrush elastomers for ultrasoft electronics
Nature Communications (2023)
-
Mucosa-interfacing electronics
Nature Reviews Materials (2022)
-
Liquid Metal Fibers
Advanced Fiber Materials (2022)