Abstract
Objectives
This post hoc analysis of pooled data from two phase III studies (AD-301: NCT02118766; AD-302: NCT02118792) explored the efficacy and safety of crisaborole ointment, 2%, a nonsteroidal phosphodiesterase 4 inhibitor, for the treatment of mild-to-moderate atopic dermatitis (AD) in pediatric patients (aged 2 to < 18 years) only, stratified by baseline characteristics.
Methods
Pediatric patients with mild or moderate AD per Investigator’s Static Global Assessment (ISGA) and percentage of treatable body surface area (%BSA) ≥ 5 at baseline were assessed. Crisaborole or vehicle (2:1 randomization ratio) was applied twice daily for 28 days. Of the 1313 pediatric patients included in this study, 874 received crisaborole and 439 received vehicle. ISGA success was defined as clear (0) or almost clear (1) with ≥ 2-grade improvement from baseline. Efficacy and safety were stratified by age group, sex, baseline ISGA, baseline %BSA per published severity strata, and prior AD therapy.
Results
Overall, the proportions of crisaborole-treated and vehicle-treated pediatric patients with ISGA success at week 4 were 32.5 and 21.5%, respectively. ISGA success rates at day 29 (week 4) were generally higher in crisaborole-treated (21.9–38.1%) than vehicle-treated (15.7–26.9%) patients across subgroups. Rates of treatment-related application site pain were 2.4–10.1% for crisaborole-treated patients and 0.6–2.2% for vehicle-treated patients across subgroups. No new safety concerns were noted in any patient subgroup.
Conclusion
Crisaborole improved global disease severity and was reasonably well tolerated across all pediatric baseline characteristic subgroups. Application site discomfort was greater with crisaborole than with vehicle, but few patients discontinued treatment.
Clinicaltrials.gov registration numbers
NCT02118766; NCT02118792 (registration date: April 21, 2014).
Plain Language Summary
Crisaborole is an ointment approved for the treatment of mild-to-moderate eczema. In two phase III clinical trials, eczema improved after 28 days of crisaborole use in patients aged ≥ 2 years. Patients with eczema rashes used crisaborole or plain ointment twice a day for 28 days. The clinical trials excluded patients with serious infections. Eczema treatment within 2 weeks of the trials was not allowed. We looked at whether traits of children aged 2–17 years affected how well crisaborole improved eczema. We studied boys and girls by age and how bad their eczema was at the start of the study. We combined data from both clinical trials to calculate the percentages of children with clear or almost clear skin at day 29. We also studied the frequency of side effects at day 29. After 4 weeks, 33% of children receiving crisaborole compared with 22% of children receiving plain ointment had clear or almost clear skin, a meaningful difference in favor of crisaborole. This was also true across groups. Most patients did not have side effects related to crisaborole. The most common side effect related to crisaborole was application site pain. This side effect occurred in up to one in ten children receiving crisaborole. Up to 1 in 50 patients receiving plain ointment had application site pain. Few children stopped crisaborole treatment, and there were no new safety concerns. In conclusion, compared with plain ointment, crisaborole improved eczema in more children, and side effects were minor.
Similar content being viewed by others
Crisaborole ointment, 2%, was effective in pediatric patients with mild-to-moderate atopic dermatitis across most baseline characteristic subgroups analyzed. |
Crisaborole was well tolerated in pediatric patients across the baseline characteristic subgroups analyzed with no new safety concerns. |
1 Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease that affects approximately 15–30% of the pediatric population and 2–10% of adults [1]. In more than 60% of pediatric patients, AD manifests before the age of 2 years [2]. Compared with adults, pediatric patients with AD more often have behavioral problems, including increased dependency, fearfulness, and sleep difficulties (which can affect social and intellectual development) [3]. Some immunological differences have also been observed between pediatric and adult patients, such as reduced counterregulation by type 1 helper T cells in pediatric patients, potentially contributing to excess type 2 helper T-cell activation [4, 5]. Despite these differences, research using a multimodal treatment model (including medical, nutritional, and behavioral support) suggested that younger age and higher baseline disease severity per Eczema Area and Severity Index are associated with treatment response [6].
Topical corticosteroids (TCSs) are considered the mainstay of AD therapy, and—in children and adolescents—mid-to-high potency TCSs may be appropriate for acute flares, with a reduction in potency as necessary for long-term use [7]. Topical calcineurin inhibitors (TCIs) are a second-line option that are effective and well tolerated in pediatric patients for continuous short-term use [8]. Notwithstanding the prescribing information boxed warning, evidence suggests that long-term use of TCIs is well tolerated [9]. However, there is still a need for effective and safe alternative treatment options for pediatric patients because patients, caregivers, and clinicians alike have concerns about the possibility of side effects with long-term use of TCS, which may impact adherence and lead to inadequate effectiveness [10, 11].
Results of in vitro studies showed that inhibition of phosphodiesterase 4 (PDE4) may decrease the inflammatory processes associated with AD [12, 13]. This led to the clinical development of PDE4 inhibitors such as crisaborole [14]. Crisaborole ointment, 2%, is a nonsteroidal PDE4 inhibitor for the treatment of mild-to-moderate AD [15]. As of January 2021, crisaborole had received regulatory approval in regions including Australia, Canada, the EU, and Israel for the treatment of mild-to-moderate AD in patients aged ≥2 years [16,17,18,19]. In the USA and Lebanon, crisaborole is approved for patients aged ≥ 3 months [15, 20]. In addition to phase II clinical studies [21,22,23], regulatory approval was based on the results of a phase IV study in infants aged 3 to < 24 months [24], two phase III studies that included adult and pediatric patients aged ≥ 2 years (AD-301: NCT02118766; AD-302: NCT02118792), and a long-term safety extension study [25, 26].
Given our specific interest in treating children and adolescents with mild-to-moderate AD and that the overall population from these two phase III studies was primarily pediatric, this post hoc analysis was undertaken using data from patients aged 2 to < 18 years to explore the consistency of crisaborole treatment effects across various baseline characteristics, such as age group (within the pediatric age range), sex, and baseline disease severity (baseline Investigator’s Static Global Assessment [ISGA], baseline percentage of treatable body surface area [%BSA]), and prior use of AD therapy.
2 Methods
2.1 Patients and Treatment
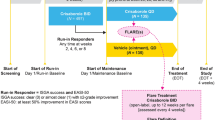
Details of the study designs have been published previously [25]. In brief, two identically designed, double-blind, randomized, vehicle-controlled trials were conducted concurrently to evaluate crisaborole compared with vehicle (2:1 randomization ratio) applied twice daily for 28 days in patients aged ≥ 2 years with AD. Participants were required to have a baseline ISGA of mild (2) or moderate (3) and baseline %BSA of ≥ 5 [25]. Patients were not eligible if they had used a TCS or a TCI within 14 days of starting the study, had a significant active infection, or had previously used biologic therapy [25]. The two studies were conducted in accordance with Good Clinical Practice Guidelines and local regulatory requirements. Quorum Review institutional review board approved the study protocols, and all participants provided informed consent.
2.2 Assessments and Outcomes
The ISGA is five-point scale used at each visit to assess overall disease severity across all treatable AD lesions [25] without reference to previous ISGA assessments (i.e., static). The Severity of Pruritus Scale (SPS) was used to assess the severity of pruritus twice daily on a four-point scale from none (0; no itching) to severe (3; bothersome itching/scratching that disturbs sleep) [27].
Endpoints in this post hoc analysis included proportion of patients achieving ISGA success (clear [0] or almost clear [1] with ≥ 2-grade improvement from baseline) at day 29 (week 4), proportion of patients achieving ISGA clear or almost clear at day 29 (week 4), proportion of patients achieving SPS success at week 4 (weekly average SPS score ≤ 1 with ≥ 1-point improvement from baseline), and safety. Only patients with an average baseline SPS score (≥ 2 assessments at day 1) and post-baseline SPS assessments were included when evaluating SPS success. Weekly SPS scores for each patient were calculated as the mean of all available post-baseline SPS scores for the patient during a corresponding week (generally up to 14 measurements). Safety endpoints included incidence of adverse events (AEs), including overall treatment-emergent AEs (TEAEs), discontinuation due to TEAEs, and application site pain.
Efficacy and safety were analyzed in the overall pediatric population (aged 2 to < 18 years) and stratified by subgroups: age (2 to < 7 years, 7 to < 12 years, 12 to < 18 years), sex, baseline ISGA (mild, moderate), baseline %BSA severity (mild [5 to < 16], moderate [16 to < 40], severe [≥ 40]) [28], and use of prior AD treatment (defined as any prior use of systemic corticosteroids, TCSs, or TCIs for the treatment of AD within 90 days before study screening).
2.3 Statistical Analysis
This post hoc analysis was performed using pooled data from the two phase III studies (AD-301 and AD-302), with data separated by individual study in a supplemental analysis. For efficacy analyses, binary endpoints were analyzed using the normal approximation to binominal proportions, i.e., assuming the observed proportion of response in each group followed a normal distribution with proportion of response to be the mean and the product of proportion of response and portion of nonresponse divided by the sample size to be the variance; hence, the differences in response rates between crisaborole and vehicle for each subgroup also followed a normal distribution, which was used to generate the 95% confidence intervals for this difference. Safety endpoints and baseline characteristics were summarized descriptively.
3 Results
3.1 Patient Characteristics
Of 1522 total patients in AD-301 and AD-302, 86.3% were aged 2 to < 18 years. Of these, 874 were randomly assigned to receive crisaborole and 439 were randomly assigned to receive vehicle. Baseline characteristics were generally balanced between treatment groups (Table 1; Table S1 in the electronic supplementary material [ESM]).
3.2 Efficacy in the Pediatric Population
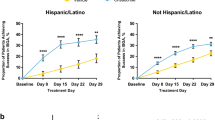
In the pooled pediatric subpopulations of AD-301 and AD-302, 32.5% of patients achieved ISGA success at week 4 in the crisaborole group compared with 21.5% in the vehicle group. Crisaborole treatment resulted in higher rates of ISGA success at week 4 compared with vehicle among all subgroups in the pediatric population (Fig. 1). Figure S1 in the ESM shows the proportions of patients achieving ISGA success by individual study. For comparison, Fig. S2 in the ESM provides major efficacy outcome results from the adult (≥ 18 years) population.
Proportions of patients achieving ISGA successa at day 29 (week 4) in the pediatric population (aged 2 to < 18 years) (AD-301 + AD-302 pooled). %BSA percentage of treatable body surface area, AD atopic dermatitis, CI confidence interval, ISGA Investigator’s Static Global Assessment. aDefined as an ISGA of clear (0) or almost clear (1) with ≥ 2-grade improvement from baseline. bDefined as any prior use of systemic corticosteroids, topical corticosteroids, or topical calcineurin inhibitors for the treatment of AD within 90 days of study screening
In the pooled pediatric populations of AD-301 and AD-302, 50.5% of patients achieved ISGA clear or almost clear at week 4 in the crisaborole group compared with 34.5% in the vehicle group. A greater proportion of crisaborole-treated patients achieved ISGA clear or almost clear at week 4 than vehicle-treated patients across all subgroups (Fig. 2). Figure S3 in the ESM shows the proportions of patients achieving ISGA clear or almost clear by individual study.
Proportions of patients achieving ISGA of clear or almost clear at day 29 (week 4) in the pediatric population (aged 2 to < 18 years) (AD-301 + AD-302 pooled). %BSA percentage of treatable body surface area, AD atopic dermatitis, CI confidence interval, ISGA Investigator’s Static Global Assessment. aDefined as any prior use of systemic corticosteroids, topical corticosteroids, or topical calcineurin inhibitors for the treatment of AD within 90 days of study screening
Among crisaborole-treated pediatric patients in AD-301 and AD-302, 34.6% achieved SPS success at week 4 compared with 20.0% of vehicle-treated patients. A greater proportion of crisaborole-treated patients achieved SPS success compared with vehicle-treated patients in all subgroups (Fig. 3). Figure S4 in the ESM shows the proportions of patients achieving SPS success by individual study.
Proportions of patients achieving SPS successa at week 4 in the pediatric population (aged 2 to < 18 years) (AD-301 + AD-302 pooled). %BSA percentage of treatable body surface area, AD atopic dermatitis, CI confidence interval, ISGA Investigator’s Static Global Assessment, SPS Severity of Pruritus Scale. aDefined as a weekly average SPS score ≤ 1 with ≥ 1-point improvement from baseline. bDefined as any prior use of systemic corticosteroids, topical corticosteroids, or topical calcineurin inhibitors for the treatment of AD within 90 days of study screening
3.3 Safety
In the overall pediatric population, 258 patients (29.6%) who received crisaborole and 115 patients (26.6%) who received vehicle reported a TEAE of any cause during the studies. Between both studies, 13 patients (1.5%) in the crisaborole arm and seven (1.6%) in the vehicle arm discontinued the studies because of an AE. Safety data for the adult (aged ≥ 18 years) population can be found in Table S2 in the ESM.
In total, 63 patients (7.2%) in the crisaborole arm and 19 (4.4%) in the vehicle arm reported a treatment-related AE. The most frequently reported treatment-related AE was application site pain (4.4 vs. 0.9%). Most treatment-related AEs were mild or moderate. The incidence of the most frequently reported treatment-related AE, application site pain, ranged from 2.4 to 10.1% in crisaborole-treated patients across subgroups and from 0.6 to 2.2% in vehicle-treated patients across subgroups and was greater with crisaborole than vehicle across all disease severities and age groups (Table 2; Table S3 in the ESM). The duration of application site pain in crisaborole-treated patients ranged widely, from 1 to 30 days with a median duration of 1 day. In vehicle-treated patients, the duration of application site pain ranged from 1 to 2 days, with a median duration of 2 days (Tables S4 and S5 in the ESM). For both crisaborole and vehicle, age group did not appear to affect the duration of application site pain.
4 Discussion
The purpose of this post hoc analysis of the pediatric population of AD-301 and AD-302 was to explore the consistency of the treatment effects across baseline characteristic subgroups. Across subgroups, more crisaborole-treated patients experienced ISGA success than vehicle-treated patients (21.9–38.1 vs. 15.7–26.9%, respectively). A similar trend was noted for the outcome of ISGA clear or almost clear (31.4–72.3 vs. 21.4–55.9%, respectively) and SPS success (29.7–37.2 vs. 16.3–25.5%, respectively). In the severe baseline %BSA subgroup (%BSA ≥ 40), crisaborole and vehicle treatment effects as measured by ISGA success and ISGA clear or almost clear were smaller, especially in AD-302. However, the relatively smaller sample size of the severe baseline %BSA subgroup compared with other subgroups tested (e.g., 158 vs. 341 and 814 patients in the mild and moderate %BSA subgroups, respectively) diminishes the reliability of the severe baseline %BSA subgroup treatment effect estimates. The treatment effect observed for patients with mild baseline ISGA for ISGA success was also smaller. This is related to the impact of the requirement for a ≥ 2-grade improvement from baseline to achieve ISGA success, which requires patients with mild baseline ISGA (2) to achieve clear ISGA (0). Overall, crisaborole appeared to be effective across baseline characteristic subgroups in pediatric patients.
In general, crisaborole was well tolerated in the pediatric population of AD-301 and AD-302. Similar to the total population presented in the primary publication [25], the most frequently reported treatment-related AE was application site pain, and this was noted broadly. No trend was observed when application site pain was analyzed by subgroup. The median duration of application site pain was 1–2 days across age groups in both the vehicle-treated and the crisaborole-treated groups. Furthermore, no new safety concerns were noted.
Crisaborole was previously evaluated in pediatric patients with mild-to-moderate AD in a phase Ib maximal-use study and a phase IIa study [21, 29]. In the phase IIa study of 23 patients aged 12–17 years, 34.8% achieved ISGA success at day 29 (week 4) [21], which is similar to the 30.3% ISGA success rate observed here in the subgroup aged 12 to < 18 years. The most frequently reported TEAEs in the phase IIa study were application site pain and nasopharyngitis (each in three patients [13%]) [21]. This rate of application site pain was relatively higher than in the subgroup aged 12 to < 18 years observed for crisaborole-treated patients in the current pooled analysis (4.4%). However, the phase IIa study comprised considerably fewer patients than the subgroup aged 12 to < 18 [21], and neither study was vehicle controlled [21, 29].
In the phase Ib maximal-use study, which consisted of 34 patients aged 2–17 years, 47.1% achieved ISGA success at day 29 (week 4), and 64.7% had ISGA clear or almost clear at day 29 (week 4) [29], which was relatively higher than the ISGA success rate and ISGA clear or almost clear rate observed in the overall pediatric population of crisaborole-treated patients in the current pooled analysis (ISGA success, 32.5%; ISGA clear or almost clear, 50.5%). However, the phase Ib study was a maximal-use trial with drug applied in fixed amounts by site staff on days 1–9 (morning doses) or 2–7 (evening doses). Regarding safety, this study was open-label with more frequent office visits (12 vs. 5) and included patients with greater %BSA involvement (i.e., ≥ 25 vs. ≥ 5) than in the phase III studies [29]. The rate of treatment-related application site pain would then be higher in the phase Ib maximal-use study (12 of 34 patients [35%]) [29] than in this analysis.
This analysis has several limitations. The analysis was post hoc, and the subgroup populations selected for these analyses were relatively small in some cases. Since the two studies were identically designed, the results were pooled to minimize the effects of the small sample size of the subgroups since the individual studies were not prospectively designed to evaluate these subgroups. The subgroup of patients with prior use of AD therapy was limited to those who had been previously treated with systemic corticosteroids, TCSs, or TCIs within the 90 days prior to study screening. Data regarding the reason for discontinuation of prior AD therapy (e.g., lack of efficacy, intolerability, unwillingness to use corticosteroids) were not collected. In addition, assessment of pruritus and application site pain is difficult in some of the youngest patients because it must be assessed and reported by an observer. In this study, no severity score was assessed by body site, and the location of the application site pain was not disclosed, so data are not available to understand whether severity was greater for more sensitive regions, including the face and neck. In addition, the strata used for assessing severity by %BSA were not prespecified for the study and were devised in adolescents and adults aged ≥ 13 years [28], so therefore have not been confirmed for use in the younger age groups in this analysis.
5 Conclusion
Crisaborole was shown to be effective and well tolerated in the pooled pediatric population of two phase III studies and across most baseline characteristic subgroups analyzed. Similar to previously reported studies in pediatric populations, application site pain was the most frequently reported treatment-related AE, and no new safety concerns were noted among the subgroups analyzed.
References
Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–37.
Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy. 2012;67(12):1475–82.
Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–9.
Leung DY. Atopic dermatitis: age and race do matter! J Allergy Clin Immunol. 2015;136(5):1265–7.
Brunner PM, Israel A, Zhang N, Leonard A, Wen HC, Huynh T, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol. 2018;141(6):P2094–106.
Chou JS, LeBovidge J, Timmons K, Elverson W, Morrill J, Schneider LC. Predictors of clinical success in a multidisciplinary model of atopic dermatitis treatment. Allergy Asthma Proc. 2011;32(5):377–83.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32.
Kalavala M, Dohil MA. Calcineurin inhibitors in pediatric atopic dermatitis: a review of current evidence. Am J Clin Dermatol. 2011;12(1):15–24.
Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016;16:75.
Nygaard U, Deleuran M, Vestergaard C. Emerging treatment options in atopic dermatitis: topical therapies. Dermatology. 2017;233(5):333–43.
Fishbein AB, Hamideh N, Lor J, Zhao S, Kruse L, Mason M, et al. Management of atopic dermatitis in children younger than two years of age by community pediatricians: a survey and chart review. J Pedriatr. 2020;221:138-44.e3.
Hanifin JM, Chan SC, Cheng JB, Tofte SJ, Henderson WR Jr, Kirby DS, et al. Type 4 phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J Invest Dermatol. 1996;107(1):51–6.
Chan SC, Li SH, Hanifin JM. Increased interleukin-4 production by atopic mononuclear leukocytes correlates with increased cyclic adenosine monophosphate-phosphodiesterase activity and is reversible by phosphodiesterase inhibition. J Invest Dermatol. 1993;100(5):681–4.
Jarnagin K, Chanda S, Coronado D, Ciaravino V, Zane LT, Guttman-Yassky E, et al. Crisaborole topical ointment, 2%: a nonsteroidal, topical, anti-inflammatory phosphodiesterase 4 inhibitor in clinical development for the treatment of atopic dermatitis. J Drugs Dermatol. 2016;15(4):390–6.
Eucrisa. Prescribing information. Pfizer Inc. 2020. https://labeling.pfizer.com/ShowLabeling.aspx?id=5331. Accessed 6 Oct 2021.
Staquis.. Prescribing information. Pfizer Australia Pty Ltd. 2019. https://apps.medicines.org.au/files/pfcstaqo.pdf. Accessed 6 Oct 2021.
Preeucrisa. Prescribing information. Pfizer Canada Inc.. 2018. https://www.pfizer.ca/sites/default/files/202107/Vyndamax_PM_EN_240239_09-Jul-2021.pdf. Accessed 6 Oct 2021.
Staquis.. Summary of product characteristics. Pfizer Europe. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/staquis. Accessed 6 Oct 2021.
Staquis.. Prescribing information. Pfizer Pharmaceuticals Israel. 2019. https://data.health.gov.il/drugs/alonim/Rishum_10_418411819.pdf. Accessed 6 Oct 2021.
Staquis.. Prescribing information. Pfizer Inc. 2020. https://labeling.pfizer.com/ShowLabeling.aspx?id=5331. Accessed 6 Oct 2021.
Tom WL, Van Syoc M, Chanda S, Zane LT. Pharmacokinetic profile, safety, and tolerability of crisaborole topical ointment, 2% in adolescents with atopic dermatitis: an open-label phase 2a study. Pediatr Dermatol. 2016;33(2):150–9.
Murrell DF, Gebauer K, Spelman L, Zane LT. Crisaborole topical ointment, 2% in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. J Drugs Dermatol. 2015;14(10):1108–12.
Bissonnette R, Pavel AB, Diaz A, Werth JL, Zang C, Vranic I, et al. Crisaborole and atopic dermatitis skin biomarkers: an intrapatient randomized trial. J Allergy Clin Immunol. 2019;144(5):1274–89.
Schlessinger J, Shepard JS, Gower R, Su JC, Lynde C, Cha A, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild-to-moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21(2):275–84.
Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494–503.e6.
Eichenfield LF, Call RS, Forsha DW, Fowler J Jr, Hebert AA, Spellman M, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2017;77(4):641–9.e5.
Yosipovitch G, Simpson EL, Bushmakin AG, Cappelleri JC, Luger T, Ständer S, et al. Assessment of pruritus in atopic dermatitis: validation of the Severity of Pruritus Scale (SPS). Itch. 2018;3:13.
Chopra R, Vakharia PP, Sacotte R, Patel N, Immaneni S, White T, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177(5):1316–21.
Zane LT, Kircik L, Call R, Tschen E, Draelos ZD, Chanda S, et al. Crisaborole topical ointment, 2% in patients 2 to 17 years of age with atopic dermatitis: a phase 1b, open-label, maximal-use systemic exposure (MUSE) study. Pediatr Dermatol. 2016;33(4):380–7.
Acknowledgements
Editorial/medical writing support under the guidance of the authors was provided by Robert J. Schoen, PharmD, and Stephanie Agbu, PhD, at ApotheCom (San Francisco, CA, USA) and was funded by Pfizer Inc., New York, NY, USA, in accordance with good publication practice (GPP3) guidelines (Ann Intern Med. 2015;163:461-464).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Pfizer Inc.
Conflict of interest
Thomas A. Luger has served as an investigator for Pfizer, AbbVie, Celgene, Eli Lilly, LEO Pharma, Menlo Therapeutics, Novartis, and Sandoz; served as a member of scientific advisory boards for Pfizer, AbbVie, Argenx, Celgene, Ceres Pharma, Galderma, Eli Lilly, Janssen-Cilag, La Roche-Posay, LEO Pharma, Menlo Therapeutics, Mylan/Meda AB, Novartis, Pierre Fabre, Piqur Therapeutics, Sandoz, Sanofi-Aventis, and Symrise; and has received funding from Pfizer, AbbVie, Celgene, Janssen-Cilag, Merck Sharp & Dohme, Mylan/Meda AB, Novartis, and Wolff Laboratories. Adelaide A. Hebert discloses that research funding was paid to UTHealth McGovern Medical School from Pfizer, Anacor, Arcutis, Cutanea, Brickell, Dermira, GlaxoSmithKline, and Novan. She has received honoraria as a member of data safety monitoring boards for Bausch, GlaxoSmithKline, and Regeneron-Sanofi and has received honoraria from Pfizer, Biofrontera, Cutanea, Dermavant, Dermira, Galderma, Eli Lilly, Leo Pharma, Ortho Dermatologics, Pierre Fabre, and Verrica. Andrea L. Zaenglein has served as an investigator for AbbVie, Arcutis, Dermavant, Incyte, and Pfizer and as a consultant for Cassiopea and Verrica. Jonathan I. Silverberg has served as an investigator for Celgene, Eli Lilly, F. Hoffmann-La Roche, Menlo Therapeutics, Realm Therapeutics, and Regeneron-Sanofi; as a consultant for Pfizer, AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermira, Dermavant, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron, and Sanofi; and as a speaker for Regeneron and Sanofi. Huaming Tan and Michael A. Zielinski are employees of and stockholders in Pfizer Inc. William C. Ports was an employee of and stockholder in Pfizer Inc. at the time of this analysis.
Availability of data and material
Upon request and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Code availability
Not applicable.
Ethics approval
This study was approved by the local medical ethical committee, and all data were processed anonymously, according to privacy legislation. All parent(s)/legal guardian(s) provided written informed consent. This study was conducted in compliance with ethical principles originating in the Declaration of Helsinki and in compliance with the International Committee on Harmonisation and Good Clinical Practice Guidelines.
Consent to participate
All parent(s) or legal guardian(s) provided written informed consent.
Consent for publication
Not applicable.
Author contributions
Thomas A. Luger, Adelaide A. Hebert, Andrea L. Zaenglein, Jonathan I. Silverberg, William C. Ports, and Michael A. Zielinski contributed to the conception and design of the analysis, the interpretation of the data, and the drafting and revision of the manuscript. Huaming Tan carried out the analysis and contributed to the interpretation of the data and the drafting and revision of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Luger, T.A., Hebert, A.A., Zaenglein, A.L. et al. Subgroup Analysis of Crisaborole for Mild-to-Moderate Atopic Dermatitis in Children Aged 2 to < 18 Years. Pediatr Drugs 24, 175–183 (2022). https://doi.org/10.1007/s40272-021-00490-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00490-y