Abstract
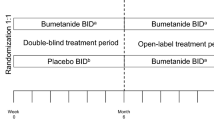
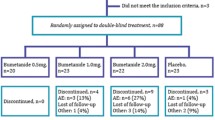
The efficacy of bumetanide (oral liquid formulation 0.5 mg bid) as a treatment for the core symptoms of autism spectrum disorders in children and adolescents aged 7–17 years is being investigated in an international, randomised, double-blind, placebo-controlled phase III study. The primary endpoint is the change in Childhood Autism Rating Scale 2 (CARS2) total raw score after 6 months of treatment. At baseline, the 211 participants analysed are broadly representative of autistic subjects in this age range: mean (SD) age, 10.4 (3.0) years; 82.5% male; 47.7% with intelligence quotient ≥ 70. Mean CARS2 score was 40.1 (4.9) and mean Social Responsiveness Scale score was 116.7 (23.4). Final study results will provide data on efficacy and safety of bumetanide in autistic children and adolescents.


Similar content being viewed by others
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®) (American Psychiatric Pub)
Chiarotti F, Venerosi A (2020) Epidemiology of autism spectrum disorders: a review of worldwide prevalence estimates since 2014. Brain Sci 10:274
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C et al (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5:160–179
Fuentes J, Basurko A, Isasa I, Galende I, Muguerza MD, García-Primo P et al (2021) The ASDEU autism prevalence study in northern Spain. Eur Child Adolesc Psychiatry 30:579–589
ASDEU (2021) Autism spectrum disorders in the European Union - prevalence. http://asdeu.eu/. Accessed 10 May 2021
Baxter AJ, Brugha T, Erskine HE, Scheurer RW, Vos T, Scott JG (2015) The epidemiology and global burden of autism spectrum disorders. Psychol Med 45:601
Oliveira G, Ataíde A, Marques C, Miguel TS, Coutinho AM, Mota-Vieira L et al (2007) Epidemiology of autism spectrum disorder in Portugal: prevalence, clinical characterization, and medical conditions. Dev Med Child Neurol 49:726–733
GBD (2016) Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 390:1211–1259
American Psychiatric Association (2020) Autism often accompanied by other conditions. Available at https://www.psychiatry.org/news-room/apa-blogs/apa-blog/2018/10/autism-often-accompanied-by-other-conditions. Accessed 10 May 2021
Fitzpatrick SE, Srivorakiat L, Wink LK, Pedapati EV, Erickson CA (2016) Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat 12:1525–1538
Schieve LA, Clayton HB, Durkin MS, Wingate MS, Drews-Botsch C (2015) Comparison of perinatal risk factors associated with autism spectrum disorder (ASD), intellectual disability (ID), and co-occurring ASD and ID. J Autism Dev Disord 45:2361–2372
van Bakel MM, Delobel-Ayoub M, Cans C, Assouline B, Jouk PS, Raynaud JP et al (2015) Low but increasing prevalence of autism spectrum disorders in a French area from register-based data. J Autism Dev Disord 45:3255–3261
Rydzewska E, Hughes-McCormack LA, Gillberg C, Henderson A, MacIntyre C, Rintoul J et al (2019) Prevalence of sensory impairments, physical and intellectual disabilities, and mental health in children and young people with self/proxy-reported autism: observational study of a whole country population. Autism 23:1201–1209
Muratori F, Turi M, Prosperi M, Narzisi A, Valeri G, Guerrera S et al (2019) Parental perspectives on psychiatric comorbidity in preschoolers with autism spectrum disorders receiving publicly funded mental health services. Front Psychiatry 10:107
Hansen BH, Oerbeck B, Skirbekk B, Petrovski B, Kristensen H (2018) Neurodevelopmental disorders: prevalence and comorbidity in children referred to mental health services. Nord J Psychiatry 72:285–291
Rosello B, Berenguer C, Baixauli I, Colomer C, Miranda A (2018) ADHD symptoms and learning behaviors in children with ASD without intellectual disability. A mediation analysis of executive functions. PLoS ONE 13:e0207286
Hollocks MJ, Jones CR, Pickles A, Baird G, Happé F, Charman T et al (2014) The association between social cognition and executive functioning and symptoms of anxiety and depression in adolescents with autism spectrum disorders. Autism Res 7:216–228
Fulceri F, Morelli M, Santocchi E, Cena H, Del Bianco T, Narzisi A et al (2016) Gastrointestinal symptoms and behavioral problems in preschoolers with autism spectrum disorder. Dig Liver Dis 48:248–254
Park HR, Lee JM, Moon HE, Lee DS, Kim B-N, Kim J et al (2016) A short review on the current understanding of autism spectrum disorders. Exp Neurobiol 25:1–13
Fuentes J, Hervás A, Howlin P (2020) ESCAP practice guidance for autism: a summary of evidence-based recommendations for diagnosis and treatment. Eur Child Adolesc Psychiatry: ePub ahead of print
Landa RJ (2018) Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry 30:25–39
Medavarapu S, Marella LL, Sangem A, Kairam R (2019) Where is the evidence? A narrative literature review of the treatment modalities for autism spectrum disorders. Cureus 11:e3901
Tonge BJ, Bull K, Brereton A, Wilson R (2014) A review of evidence-based early intervention for behavioural problems in children with autism spectrum disorder: the core components of effective programs, child-focused interventions and comprehensive treatment models. Curr Opin Pychiatry 27:158–165
Perihan C, Burke M, Bowman-Perrott L, Bicer A, Gallup J, Thompson J et al (2020) Effects of cognitive behavioral therapy for reducing anxiety in children with high functioning ASD: a systematic review and meta-analysis. J Autism Dev Disord 50:1958–1972
Fung LK, Mahajan R, Nozzolillo A, Bernal P, Krasner A, Jo B et al (2016) Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics 137:S124–S135
EMA (2017) Haldol summary of product characteristics. https://www.ema.europa.eu/en/documents/referral/haldol-article-30-referral-annex-iii_en.pdf. Accessed 10 May 2021
EMA (2018) Slenyto summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/slenyto-epar-product-information_en.pdf. Accessed 10 May 2021
Cellot G, Cherubini E (2014) GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr 2:70
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K et al (1999) The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255
Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3:728–739
FDA (2009) BUMEX bumetanide tablets packaging label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018225s024lbl.pdf. Accessed 10 May 2021
Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA et al (2005) NKCC1 transporter facilitates seizures in the developing brain. Nat Med 11:1205–1213
Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R (2007) GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev 87:1215–1284
Lemonnier É, Degrez C, Phelep M, Tyzio R, Josse F, Grandgeorge M et al (2012) A randomised controlled trial of bumetanide in the treatment of autism in children. Transl Psychiatry 2:e202–e202
Lemonnier E, Villeneuve N, Sonie S, Serret S, Rosier A, Roue M et al (2017) Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl Psychiatry 7:e1056–e1056
Crutel V, Lambert E, Penelaud P-F, Severo CA, Fuentes J, Rosier A et al (2021) Bumetanide oral liquid formulation for the treatment of children and adolescents with autism spectrum disorder: design of two Phase III studies (SIGN trials). J Autism Dev Disord 51:2959–2972
Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S (2012) Autism Diagnostic Observation Schedule–Second Edition (ADOS-2) Western Psychological Corporation (Los Angeles, CA, USA)
Lord C, Rutter M, Le Couteur A (1994) Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685
Guy W (1976) ECDEU assessment manual for psychopharmacology (US Department of Health, Education, and Welfare, Public Health Service)
Schopler E, Reichler RJ, Renner BR (2010) The childhood autism rating scale (CARS) (WPS, Los Angeles, CA)
Constantino JN, Gruber CP (2012) Social responsiveness scale: SRS-2 (WPS, Torrance, CA)
Busner J, Targum SD (2007) The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry 4:28–37
European Medicines Agency (2017). Guideline on the clinical development of medicinal products for the treatment of Autism spectrum disorder (ASD). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-medicinal-products-treatment-autism-spectrum-disorder-asd_en.pdf. Accessed 19 Nov 2021
Varni JW, Seid M, Rode C (1999) PedsQL: measurement model for the pediatric quality of life inventory. Med Care 37:126–139
WHO (1996) World Health Organization - WHOQOL-BREF: introduction, administration, scoring, and generic version of the assessement. Field trial version: December 1996. https://www.who.int/mental_health/media/en/76.pdf. Accessed 10 May 2021
SAS (2008) SAS Institute. SAS PC software, version 9.2 of the SAS system for Windows. Cary, NC: SAS Institute; 2008
WHO (2007) World Health Organization - growth reference data for 5–19 year olds. https://www.who.int/growthref. Accessed 10 May 2021
Howes OD, Rogdaki M, Findon JL, Wichers RH, Charman T, King BH et al (2018) Autism spectrum disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J Psychopharmacol 32:3–29
NICE (2014) National Institute for Health and Care Excellence - antipsychotic medication. https://www.nice.org.uk/guidance/cg178/ifp/chapter/Antipsychotic-medication. Accessed 10 May 2021
Lemonnier E, Ben-Ari Y (2010) The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta Paediatr 99:1885–1888
Murray ML, Hsia Y, Glaser K, Simonoff E, Murphy DG, Asherson PJ et al (2014) Pharmacological treatments prescribed to people with autism spectrum disorder (ASD) in primary health care. Psychopharmacology 231:1011–1021
Bachmann CJ, Gerste B, Hoffmann F (2018) Diagnoses of autism spectrum disorders in Germany: time trends in administrative prevalence and diagnostic stability. Autism 22:283–290
Delobel-Ayoub M, Saemundsen E, Gissler M, Ego A, Moilanen I, Ebeling H et al (2020) Prevalence of autism spectrum disorder in 7-9-year-old children in Denmark, Finland, France and Iceland: a population-based registries approach within the ASDEU project. J Autism Dev Disord 50:949–959
Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z et al (2018) Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 67:1–23
Bickel J, Bridgemohan C, Sideridis G, Huntington N (2015) Child and family characteristics associated with age of diagnosis of an autism spectrum disorder in a tertiary care setting. J Dev Behav Pediatr 36:1–7
Brett D, Warnell F, McConachie H, Parr JR (2016) Factors affecting age at ASD diagnosis in UK: no evidence that diagnosis age has decreased between 2004 and 2014. J Autism Dev Disord 46:1974–1984
Salomone E, Charman T, McConachie H, Warreyn P (2016) Child’s verbal ability and gender are associated with age at diagnosis in a sample of young children with ASD in Europe. Child Care Health Dev 42:141–145
Wiggins LD, Baio J, Rice C (2006) Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr 27:S79-87
Mandell DS, Morales KH, Xie M, Lawer LJ, Stahmer AC, Marcus SC (2010) Age of diagnosis among Medicaid-enrolled children with autism, 2001–2004. Psychiatr Serv 61:822–829
Mazurek MO, Handen BL, Wodka EL, Nowinski L, Butter E, Engelhardt CR (2014) Age at first autism spectrum disorder diagnosis: the role of birth cohort, demographic factors, and clinical features. J Dev Behav Pediatr 35:561–569
Muskens JB, Velders FP, Staal WG (2017) Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: a systematic review. Eur Child Adolesc Psychiatr 26:1093–1103
Hossain MM, Khan N, Sultana A, Ma P, McKyer ELJ, Ahmed HU et al (2020) Prevalence of comorbid psychiatric disorders among people with autism spectrum disorder: an umbrella review of systematic reviews and meta-analyses. Psychiatry Res 287:112922
Madden JM, Lakoma MD, Lynch FL, Rusinak D, Owen-Smith AA, Coleman KJ et al (2017) Psychotropic medication use among insured children with autism spectrum disorder. J Autism Dev Disord 47:144–154
Sprengers JJ, Van Andel DM, Zuithoff NP, Keijzer-Veen MG, Schulp AJ, Scheepers FE et al (2021) Bumetanide for core symptoms of autism spectrum disorder (BAMBI): a single center, double-blinded, participant-randomized, placebo-controlled, Phase-2 superiority trial. J Am Acad Child Adolesc Psychiatry 60:865–876
Zhang L, Huang CC, Dai Y, Luo Q, Ji Y, Wang K et al (2020) Symptom improvement in children with autism spectrum disorder following bumetanide administration is associated with decreased GABA/glutamate ratios. Transl Psychiatry 10:9
Greenberg RG, Gamel B, Bloom D, BradleyJ JHS, Hinton D et al (2018) Parents’ perceived obstacles to pediatric clinical trial participation: findings from the clinical trials transformation initiative. Contemp Clin Trials Commun 9:33–39
Caldwell PH, Murphy SB, Butow PN, Craig JC (2004) Clinical trials in children. Lancet 364:803–811
Klassen TP, Hartling L, Hamm M, van der Leer JH, Offringa M (2009) StaR Child Health: an initiative for RCTs in children. Lancet 374:1310–1312
Acknowledgements
We thank the patients and their families for their participation in this study.
Funding
This study was supported by Servier. Medical writing support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing and referencing) was provided by Fiona Goodwin and Rebecca Cunningham of Aura, a division of Spirit Medical Communications Group Limited (Manchester UK) and was funded by Servier.
Author information
Authors and Affiliations
Contributions
MF, CG, BPK, AH, SM, GO, AR, and JF acquisition of data—critical review and revision of the manuscript—approval of final version for submission. VC, EB, CAS, DR, study conception and design—data analysis and interpretation—critical review and revision of the manuscript—approval of final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
Maite Ferrin—research support from Servier; advisory board participation for Novartis. Christina Georgoula, Bozena Pietraszczyk-Kedziora, Amaia Hervas, Stéphane Marret, Guiomar Oliveira, Antoine Rosier—research support from Servier. Véronique Crutel, Emmanuelle Besse, Cristina Albarrán Severo—employee of Servier. Denis Ravel—employee of Neurochlore. Joaquin Fuentes—research support from Policlínica Gipuzkoa Foundation, Servier and AIMS-2-Trials project ID 777394. Partial support for attending professional meetings from Policlínica Gipuzkoa Foundation, ESCAP and AACAP.
Research Involving Human Participants
All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Georgoula, C., Ferrin, M., Pietraszczyk-Kedziora, B. et al. A Phase III Study of Bumetanide Oral Liquid Formulation for the Treatment of Children and Adolescents Aged Between 7 and 17 Years with Autism Spectrum Disorder (SIGN 1 Trial): Participant Baseline Characteristics. Child Psychiatry Hum Dev 54, 1360–1372 (2023). https://doi.org/10.1007/s10578-022-01328-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10578-022-01328-5