Abstract
Blood vessel endothelial cells (ECs) have long been known to modulate inflammation by regulating immune cell trafficking, activation status and function. However, whether the heterogeneous EC populations in various tissues and organs differ in their immunomodulatory capacity has received insufficient attention, certainly with regard to considering them for alternative immunotherapy. Recent single-cell studies have identified specific EC subtypes that express gene signatures indicative of phagocytosis or scavenging, antigen presentation and immune cell recruitment. Here we discuss emerging evidence suggesting a tissue-specific and vessel type-specific immunomodulatory role for distinct subtypes of ECs, here collectively referred to as ‘immunomodulatory ECs’ (IMECs). We propose that IMECs have more important functions in immunity than previously recognized, and suggest that these might be considered as targets for new immunotherapeutic approaches.
Similar content being viewed by others
Introduction
Blood vessels had long been viewed as passive bystander conduits, with their sole function being the supply of blood to and the drainage of blood from organs. Whereas lymph vessels are known to regulate various aspects of immunity1,2, a potentially similar role for blood vessels has not received sufficient attention to date. Interestingly, endothelial cells (ECs), the cells that line blood vessels, share a common ancestor with immune cells (Box 1), intuitively supporting a role for ECs in immune responses.
Research from more than 100 years ago showed that ECs from the sinusoids of the liver, spleen and other organs can act as scavenger ECs, complementing the activity of macrophages in eliminating circulating waste macromolecules3,4. Indeed, scavenger ECs were proposed 10 years ago to be “an integral component of the innate immune system”3, and like immune cells, liver sinusoidal ECs (LSECs) in rats can arise from bone marrow precursors in response to liver injury and during liver regeneration3. In addition, a combined single-cell RNA sequencing (scRNA-seq) and single-cell assay for transposase-accessible chromatin sequencing study identified an “immune cell-like EC (EndICLT)” subpopulation among mouse aortic ECs, which is induced by disturbed blood flow. Induction of EndICLT marker genes was confirmed in vitro in human aortic ECs under disturbed flow-mimicking conditions5. In addition, it was found that during mouse embryonic development, aortic ECs can bud off from the ventral aorta and transition into haematopoietic cells; this was in part dependent on the transcription factor RUNX1 (ref.6). Moreover, adult mouse ECs can be reprogrammed in vivo into haematopoietic stem cell-like cells through transient expression of the transcription factors FOSB, GFIL, RUNX1 and SPI1, and vascular-niche derived angiocrine factors7.
Emerging evidence indicates that subsets of ECs in different tissues and organs exert immunomodulatory activities beyond their well-known role in alloimmunity, immune cell recruitment, immune tolerance and vascular inflammation8,9,10. Furthermore, several subtypes of ECs have been shown to display features that are typical of immune cells. These include the expression of co-stimulatory and co-inhibitory receptors11, the capacity to induce apoptosis in other cells (for example, they have been shown to kill ovarian tumour-homing cytotoxic T cells via FAS ligand (FASL) in human co-cultures and mice12), secretion of cytokines and their acting as (semi-professional) antigen-presenting cells (APCs). They can also act as phagocytes and scavengers of circulating waste macromolecules and participate in efferocytosis4,11,12,13,14. Notably, immunomodulation by ECs can be influenced by cytokines, such as interleukin-35 (IL-35)15 and IL-17A16. Given that ECs are among the first cells to come into contact with circulating pathogens and are the first cells that immune cells interact with when invading tissue parenchyma, they are strategically ideally positioned as a first-line defence system to participate in immune responses.
In this Perspective, we first provide an overview of some of the well-known ‘traditional’ immunomodulatory functions of ECs, such as immune cell recruitment and semi-professional antigen presentation. We then examine recent advances in our understanding of the context-dependent role of ECs in immunomodulation in different organs, which are based mainly on scRNA-seq analyses. These studies indicate that immunomodulation by specific subsets of ECs, which we collectively refer to as ‘immunomodulatory ECs’ (IMECs), can have a prominent role in tissue-specific immunity, as well as in cancer, neurodegeneration and infectious diseases such as COVID-19. Some of these IMECs may have constitutive immunomodulatory activities (such as LSECs), while other IMECs may refer to (transitory) plastic phenotypes, induced by particular contextual conditions (such as EndICLTs).
Immune cell recruitment by ECs
In the late 1990s and early 2000s, ECs were discovered to function as local gatekeepers of immunity8. By interacting with circulating innate and adaptive immune cells and controlling their extravasation from the circulation into the tissue parenchyma, ECs can indeed control tissue and lymph node inflammation11,17. This process involves the differential expression of adhesion molecules (such as vascular cell adhesion molecule 1 (VCAM1)), selectins (such as E-selectin and P-selectin), addressins (such as peripheral node addressins, mostly in mucosal and lymphoid tissue) and chemokines (such as CCL2 and CXCL10) by ECs. During immune homeostasis, they allow patrolling immune cells to extravasate into tissue, and during inflammation, ECs can become activated and capable of actively recruiting effector immune cells11,18. EC activation can be induced by cytokines such as IL-6, IL-1β and tumour necrosis factor (TNF), but also by pathogen-associated molecular patterns, such as lipopolysaccharide19,20. The surface repertoire of adhesion molecules, selectins and addressins on ECs as well as their repertoire of secreted chemokines, in combination with the differential expression of cognate integrins, selectin ligands and chemokine receptors by immune cells, determines which circulating immune cells invade which tissue21. Some aspects of immune cell recruitment by ECs might differ between species (as is also the case for antigen presentation (see the next section and Box 2)).
Antigen presentation by ECs
Some EC subtypes are considered semi-professional APCs as they express genes involved in antigen capture, processing and presentation. For example, human renal vascular ECs express the major histocompatibility complex class II (MHC-II) surface molecule HLA-DR, which allows them to present antigens to CD4+ T cells22,23,24, and in vitro experiments showed that human umbilical vein ECs can activate allogenic T cells22,23,24,25. However, unlike professional APCs (such as dendritic cells), ECs generally do not express the surface receptors CD80 and CD86 (ref.26), which bind to CD28 on naive T cells and are required for their activation. ECs therefore primarily activate antigen-experienced T cells, although experiments in mice have shown that naive T cells can also be activated by ECs in the context of alloimmunity27,28. Importantly, not all molecules/processes related to APC function in ECs are conserved between species29 (Box 2). Interferon-γ (IFNγ) and TNF induce immunomodulatory processes in human and mouse ECs in vitro, including antigen uptake, processing and presentation9,10. Antigen presentation and immune cell recruitment by ECs contribute to alloimmunity and kidney/heart transplantation failure, for example through CD8+ T cell-induced lysis of ECs in the donor tissue8,30,31,32,33. Moreover, antigen presentation by human ECs has been implicated in autoimmune diseases such as rheumatoid arthritis34.
There are estimated to be more than 1013 ECs in the human body35; thus, even if only a fraction of ECs acts as semi-professional APCs, they form a large reservoir of potential APCs. ECs contextually present intracellular and extracellular antigens depending on the EC subtype and activation status9,36. For the presentation of intracellular antigens by ECs, nitric oxide37 and IFNγ can induce a modified proteasome38,39, called the ‘immunoproteasome’, which facilitates antigen degradation and antigen loading39. ECs share many features with professional APCs, but differ from them in other aspects (Table 1). For instance, ECs are exposed to shear stress40, which has been found to increase intercellular adhesion molecule 1 (ICAM1) expression41,42,43. ICAM1 binds to T cell integrins, which are capable of increasing T cell receptor signalling44. Moreover, shear stress increases the binding of selectins45,46,47, upregulates E-selectin expression in response to IL-1β48 and inhibits E-selectin expression in response to TNF42. Through binding to P-selectin glycoprotein ligand 1 on T cells, E-selectin can increase T cell receptor signalling, co-inhibitory molecule expression and T cell proliferation in the context of antigen presentation by ECs49. The roles of non-conventional MHC molecules such as MR1 (activating mucosal-associated invariant T cells50) and BTN3A1 (presenting phosphoantigens to Vγ9Vδ2+ T cells51) in antigen presentation in ECs have yet to be determined.
Tissue-specific immunomodulation by ECs
Studies from the past two decades examined possible roles of ECs in immunomodulation at the bulk population level11,18,35,52,53,54. A recent transcriptomic and epigenomic study on bulk mouse ECs reported tissue-specific patterns of gene transcription, with notable differences in expression patterns of co-stimulatory molecules as well as chemokines and cytokines, suggesting tissue-specific immunomodulation by ECs55. Single-cell studies have now allowed deeper insights to be obtained into the role of EC immunomodulation in (1) the recruitment and homing of immune cells to lymph nodes, (2) the modulation of immunity in response to external challenges in the liver and lung, (3) the detection and clearance of immune complexes in the liver and kidney and (4) the shielding of the brain tissue parenchyma from immune cell invasion in healthy conditions.
Lymphoid organs
Secondary lymphoid organs, such as lymph nodes and Peyer patches, and tertiary lymphoid organs that arise in response to chronic inflammation are of particular interest in the context of immunomodulation by ECs, as these form ‘hubs’ in the lymphatic system where cells of the innate and adaptive immune systems interact56. Lymph nodes contain a vascular bed with a heterogeneous composition of ECs that line arterioles, capillaries and venules. Notably, lymph nodes also contain high endothelial venules (HEVs); these are a subtype of postcapillary venules (PCVs) that are lined by high (tall and plump) ECs that are specialized in recruiting immune cells such as monocytes, plasmacytoid dendritic cell precursors, neutrophils, B cells and T cells17,57,58,59. Naive T cells in the circulation home to lymph nodes, a process that, under non-inflamed conditions, is mediated by the adhesion molecule L-selectin, which binds to addressins on HEVs. These include adhesion molecules such as CD34, podocalyxin, GLYCAM1 or MADCAM1 containing the 6-sulfo sialyl Lewis X glycan modification. These modified adhesion molecules can be detected by antibodies binding peripheral node addressins, such as MECA-79 (refs60,61,62). A combination of addressins and chemokines such as CCL21 facilitates the capture and tethering of naive T cells on HEVs and promotes their extravasation17 (Fig. 1a). HEVs are extensively remodelled upon infection and the subsequent expansion of draining lymph nodes17, but their phenotypic plasticity is only beginning to be explored.
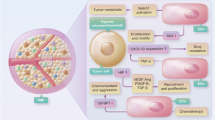
Known and putative insights into immunomodulation by endothelial cells (ECs) in lymph nodes and the liver. a | Lymph nodes contain high endothelial venules (HEVs), which express chemokines, adhesion molecules and other surface molecules (addressins) that facilitate the adhesion or recruitment of lymphocytes such as naive T cells (Tn cells). b | During inflammation (indicated by the red background), HEVs (upper panel) and venous ECs (bottom panel) in lymph nodes can recruit various immune cells, such as neutrophils, monocytes and effector T cells, in a selectin-dependent manner. c | In preclinical models of cancer, including breast cancer, melanoma that has metastasized to the lung and pancreatic cancer, anti-angiogenic therapy (AAT) or delivery of LIGHT protein, combined with immune checkpoint blockade (ICB), was found to increase HEV biogenesis, thereby promoting tumour immunity and immunotherapy78,79,80. d | Interestingly, activated HEVs express additional immunomodulatory genes, which may impair dendritic cell (DC) activation (via reverse CD137–CD137L signalling)69, alter macrophage differentiation (via macrophage migration inhibitory factor (MIF)70,71) or inhibit T cell activation (via thrombospondin 1 (TSP1)72). e | Liver ECs with immunomodulatory properties (these are mostly liver sinusoidal ECs (LSECs)) facilitate tolerance to harmless gut flora-derived antigens through co-inhibition of CD8+ T cells via the checkpoint ligand programmed death ligand 1 (PDL1) upon cross-presentation of gut flora-derived antigens via major histocompatibility complex (MHC) class I or through the induction of regulatory T cells (Treg cells) (upon presentation of gut flora-derived antigens to CD4+ T cells by MHC class II). f | LSECs clear immune complexes from the circulation via uptake and degradation. g | Periportal LSECs sense gut bacteria and recruit resident macrophages and lymphocytes through chemokine gradients. Besides zone-specific immunomodulation, LSECs might form a hub for communication with resident macrophages through cytokine signalling, thereby altering macrophage phenotypes in a context-dependent manner. h | In hepatocellular carcinoma, malignant hepatocyte-derived vascular endothelial growth factor (VEGF) induces plasmalemma vesicle-associated protein-positive (PLVAP+) tumour ECs (TECs) to form an immunosuppressive niche of folate receptor-β-positive (FOLR2+) macrophages and Treg cells. Therapeutic approaches that break LSEC-mediated immune tolerance can impair liver metastasis in preclinical models of metastatic melanoma, breast carcinoma and colon carcinoma97. i | In regions of liver fibrosis, atypical chemokine receptor 1-positive (ACKR1+) ECs might recruit and modulate/polarize macrophages through the secretion of differentiation factors such as the protein GAS6, growth arrest-specific protein 6 (GAS6) in a contextual manner. Asterisks indicate recent insights which we considered novel for immunomodulatory EC biology. TCR, T cell receptor.
An outstanding question is whether the interaction between HEVs and immune cells is sufficiently long to allow immunomodulation by the ECs. For T cells, which can reside in lymph node ‘pockets’ close to HEVs17, the interactions may be long enough to allow HEVs to modulate T cell activity and differentiation through the expression of co-inhibitory or co-stimulatory receptors and the secretion of cytokines. However, this might be a T cell/HEV-specific phenomenon, given that transendothelial migration of immune cells across conventional PCVs, which are the primary site of immune cell recruitment in many organs, is rapid63,64,65 (for example, 6 min for mouse neutrophils in vivo66), which limits sustained interactions with ECs. In the liver, lungs and kidneys, however, immune cell recruitment occurs primarily in capillaries, which are often only a few micrometres in diameter63,67. This causes immune cells to crawl, slows down extravasation and prolongs interactions with ECs, potentially allowing immunomodulation by ECs.
The characterization of HEVs at single-cell resolution under inflammatory conditions has strengthened the concept that HEVs can modulate immune cells (Fig. 1d). Indeed, scRNA-seq analysis of enriched mouse MECA-79+ HEVs from lymph nodes, isolated after oxazolone-induced inflammation (which promotes HEV activation68), revealed an upregulation of EC activation markers and the co-stimulatory molecule CD137, which can suppress the activation of immune cells that express CD137L such as dendritic cells69. Activated HEVs from oxalozone-exposed mice also express higher levels of macrophage migration inhibitory factor (MIF), which regulates context-dependent M1/M2 macrophage polarization70,71, and thrombospondin 1 (TSP1), which can impair T cell activation72. Together, these findings suggest that HEVs have immunomodulatory functions beyond immune cell recruitment73. Another scRNA-seq study of mouse lymph nodes implied that non-HEV ECs can recruit myeloid cells to lymph nodes during inflammation in a MECA-79-independent, but P-selectin and E-selectin-dependent manner74, implying that not only HEVs are important for (myeloid) immune cell recruitment during inflammation (Fig. 1b). Single-cell studies in mouse and human tumours further revealed that there is no clear phenotypic separation between HEVs and (postcapillary) venous ECs in tumours, which express a selected set of canonical and non-canonical HEV markers75,76,77.
Interestingly, a combination therapy consisting of anti-VEGF therapy (which facilitates vessel normalization) and anti-PDL1 immunotherapy promotes HEV formation and T cell recruitment, and improves antitumour immunity in preclinical tumour models78. Similarly, the treatment of mice with anti-PD1 in combination with delivery of vascular-targeted LIGHT proteins that induce non-canonical NF-κB signalling, which is required for differentiation of ECs into the HEV phenotype, induces HEV biogenesis and improves tumour immunity and immunotherapy in preclinical tumour models79,80 (Fig. 1c). Thus, in addition to the established function of HEVs in immune cell trafficking to lymph nodes during infections, HEVs may also have direct immunomodulatory effects. Further insight into this additional immunomodulatory potential and the extralymphatic biogenesis of HEVs during (chronic) inflammation, cancer and other diseases may offer new immunotherapeutic opportunities for these conditions.
Organs controlling immunity versus tolerance to external danger
Several organs, such as the liver, intestines, lung and skin, are exposed to airborne or nutrient-derived antigens, pathogens and toxins and to their microbiota, as well as microbiota-derived antigens (Fig. 1e). These organs must both protect the organism against harmful attacks by raising an adequate immune response and, at the same time, prevent uncontrolled or excessive immune attacks against harmless agents by inducing tolerance — a delicate balance that requires fine-tuned immunoregulation.
The liver
The liver is exposed to microbial and dietary antigens from the gut via the portal vein. Specialized EC subpopulations in the liver contribute to immune tolerance, most notably LSECs. LSECs are equipped with a repertoire of molecules for the detection and uptake of extracellular antigens (microbial products and viruses), including Toll-like receptor 1 (TLR1), TLR2, TLR3, TLR4, TLR6, TLR8, TLR9 (refs81,82) and scavenger receptors such as the C-type lectin receptor mannose receptor83,84. In mice, LSECs take up and cross-present extracellular antigens on MHC-I molecules to CD8+ T cells, but have a tolerogenic function because they express high levels of co-inhibitory molecules such as PDL1 and do not express (or express at only low levels) the co-stimulatory receptors CD80 and CD86, which are necessary for the activation of naive T cells85,86,87. Similarly, exogenous antigens, acquired through mannose receptor-mediated endocytosis and presented on MHC-II molecules to naive CD4+ T cells, induce tolerance by promoting differentiation of regulatory T cells (Treg cells)88,89 (Fig. 1e). Additionally, LSECs are also involved in Fc receptor-mediated phagocytosis and degradation of (primarily large) antibody–antigen immune complexes from the circulation3,90 (Fig. 1f).
LSECs recruit different immune cells via different molecular mechanisms. For example, Treg cells migrate through the liver sinusoidal endothelium primarily by interacting with the scavenger receptor stabilin 1 and the adhesion molecules ICAM1 and VAP1, whereas CD8+ T cell extravasation into the liver is mediated primarily by ICAM1 (refs91,92,93). As LSECs exhibit zone-dependent heterogeneity in liver lobules94,95, these findings raise the question of whether LSEC heterogeneity might contribute to zone-specific recruitment of Treg cells and accompanying immunosuppression in the liver. A recent study showed that resident myeloid and lymphoid cells cluster around periportal hepatic zones96 owing to MYD88-dependent signalling in LSECs. This is induced by gut commensal bacteria and changes the composition of the LSEC glycocalyx layer and hence the gradients of chemokines (such as CXCL9) binding to components of the glycocalyx (such as glycosaminoglycans) (Fig. 1g). The resulting periportal concentration of immune cells was more efficient than a uniform distribution of immune cells in protecting against systemic bacterial dissemination. This demonstrates that LSECs actively orchestrate the localization of immune cells, which optimizes host defence.
However, single-cell studies revealed confounding results. Indeed, the transcriptome of periportal LSECs differs from that of central vein LSECs in the human liver. Central vein LSECs upregulate the expression of CD32B (also known as FCGR2B; encoding an inhibitory receptor) and STAB1 (encoding stabilin 1) and of genes involved in innate immunity, phagocytosis and leukocyte activation, whereas periportal ECs exhibit a TNF activation signature and express other immunomodulatory genes95. However, a paired-cell RNA-seq study of livers from healthy mice, in which mRNA from pairs of ECs attached to hepatocytes was sequenced and gene expression from one cell type was used to infer the tissue coordinates of the cell pair, reported opposite findings, indicating low levels of STAB1 transcription in central vein LSECs94. Moreover, this report identified close interactions between LSECs and Kupffer cells (liver-resident macrophages) through colony-stimulating factor 1 (CSF1)–CSF1 receptor and CD93–C1qa signalling94 (Fig. 1g). Overall, although all these studies documented regional LSEC heterogeneity and interactions between LSECs and immune cells, further protein-level validation is needed to confirm their relevance.
LSECs also affect disease outcome. For example, LSECs present cancer cell-derived apoptotic bodies to naive CD8+ T cells. However, as LSECs act as semi-professional APCs, they impair the differentiation of naive CD8+ T cells into cytotoxic effector T cells, which are capable of killing cancer cells, thereby hampering tumour immunity1. It was shown that breaking LSEC-induced immune tolerance (using nanoparticles to deliver melittin, a host defence peptide with immunomodulatory activity) leads to LSEC activation and a changed hepatic chemokine and cytokine milieu, which inhibits metastasis in melanoma, breast cancer and colon cancer models97. In mouse models of hepatocellular carcinoma, malignant hepatocyte-derived VEGF induces the expression of the EC-specific transmembrane protein PLVAP in LSECs, which promotes the recruitment of FOLR2+ immunosuppressive tumour-associated macrophages and the creation of an immunosuppressive niche by interacting with Treg cells98. This suggests that LSECs form a communication hub in the liver tumour microenvironment that promotes immunosuppression and thereby facilitates tumour growth (Fig. 1h).
LSECs can also promote excessive inflammation in mice and humans and contribute to organ damage in conditions such as autoimmune hepatitis99,100 and fibrosis101, suggesting that immunomodulation by LSECs is critical for maintaining an immunological balance and tissue homeostasis in the liver. Furthermore, an scRNA-seq study of healthy and cirrhotic human livers showed that the latter contained a disease-specific EC population in the fibrotic niche101, which was enriched in ACKR1 transcripts101 (Fig. 1i), encoding the atypical chemokine receptor 1 (ACKR1). This chemokine receptor is primarily expressed by PCV ECs (and small venule ECs102), and transports basal chemokines for presentation at the luminal surface of ECs and in paracellular junctions, where it regulates different stages of immune cell diapedesis103 and recruitment104. Moreover, in silico analyses predicted that ACKR1+ ECs interact with disease-specific macrophages via multiple chemokines (such as CXCL12 and CCL2) and the macrophage differentiation factors GAS6 and PROS1 (ref.101). This suggests that ACKR1+ ECs might recruit disease-specific immune cells, and raises the question of whether liver ECs might be therapeutic targets to treat cirrhosis. In mice with experimentally induced portal hypertension, LSECs express lower levels of MHC-I and MHC-II molecules105, suggesting that immune responses in the liver may be altered in this disease. Finally, an scRNA-seq study in aged mice revealed decreased expression of Mrc1 (encoding the C-type lectin receptor CD206) in LSECs, which might contribute to their decrease in endocytic capacity with age106. However, in situ RNA staining for Mrc1 and the classical LSEC marker Pecam1 (encoding CD31) in the same study showed that the number of Mrc1-expressing LSECs actually increases with age in mice, raising the question of whether LSECs in aged individuals have a reduced or a similar immunomodulatory potential. Overall, LSECs differ from ECs in other tissues by their constant exposure to dietary and pathogen-derived antigens, exert a predominantly tolerogenic APC function and show zonal heterogeneity.
The lung
The lung is highly vascularized with a specialized composition of ECs, consisting largely of microvascular ECs that facilitate gas exchange between the circulation on the apical side and the air in alveoli on the basal side. Inhalation of large volumes of air exposes the lung to pathogens and pollutants, to which appropriate immune responses are required that do not put the vital gas exchange apparatus at risk. The lung has elaborate mechanisms to ensure homeostasis and dampen immune activation following lung damage107. Immunomodulation by ECs might play a more important role in the lung than originally anticipated.
Indeed, compared with mouse ECs from the heart or brain, the gene expression signature as detected by bulk RNA-seq of lung ECs showed a marked upregulation of transcripts involved in immune regulation108. Moreover, subsets of lung ECs express MHC-II, and in humans this feature appears to be restricted to capillary ECs75,109. A recent scRNA-seq study revealed that human bronchial ECs form a transcriptomically distinct population from alveolar ECs, although the genes involved in immunomodulation do not appear to be their most distinguishing feature110. Another single-cell study suggested that human alveolar capillary ECs can be divided in two populations on the basis of their transcriptome and location, where ECs termed ‘aerocytes’ (which are located close to alveolar type 1 epithelial cells) are specialized in gas exchange and immune cell recruitment, whereas general capillary ECs can activate CD4+ T cells through MHC-II (ref.111), suggesting that these alveolar ECs might facilitate an adequate immune response against harmful antigens.
Though yet to be confirmed, VEGF may contribute to preventing uncontrolled, detrimental immune responses to the (commensal) microbiota (Fig. 2c). Indeed, a single-cell analysis of alveolar cell populations (conserved in humans, mice, rats and pigs) predicted capillary ECs to be the cell type most responsive to VEGF (released primarily by alveolar type 1 cells and secretory epithelial cells112). Given the immunosuppressive effects of VEGF113, the aforementioned finding raises the question of whether VEGF signalling in the alveolar microenvironment might contribute to EC-mediated tolerance to airborne pathogens and toxins in the lung. Whether additional molecular mechanisms contribute to the tolerogenic nature of lung ECs with immunomodulatory features requires further study.
Known and putative insights into endothelial cell (EC) immunomodulation per tissue type. a | In lung cancer, tumour ECs (TECs) are generally immunosuppressive as they display decreased expression levels of antigen-presenting molecules, intercellular adhesion molecule 1 (ICAM1) and various cytokines and chemokines compared with normal lung ECs. Further immunosuppressive features of lung TECs include the elevated expression of FAS ligand (FASL), which induces CD8+ T cell apoptosis, and high levels of inhibitory molecules such as PDL1. By contrast, chronic tumour inflammation (indicated by a red background) induces pro-inflammatory high endothelial venule (HEV)-like ECs, which can also occur in other tissues with chronic inflammation. b | In malaria, specific lung immunomodulatory ECs (IMECs) take up and present parasite antigens to CD8+ T cells, which then kill ECs by cytolysis, leading to vascular leakage and lung damage. c | Lung IMECs in alveoli are involved in immune cell recruitment and in controlling a delicate balance between immunity and tolerance to pathogens through high expression of major histocompatibility complex (MHC) class II. This possibly involves vascular endothelial growth factor (VEGF), which has an immunosuppressive function; however, the exact underlying mechanisms require further investigation. d | Glomerular ECs with a particularly thick glycocalyx (as depicted, although other ECs generally also have a glycocalyx, which is not shown) impair immune cell infiltration by shielding adhesion/selectin molecules (here represented by cell adhesion molecules (CAMs), which include mainly but not exclusively integrin ligands) on their surface (therefore not visible in the figure). In kidney disease (indicated by the red background), glycocalyx shedding exposes these molecules and promotes immune cell recruitment and inflammation. e | Glomerular ECs clear immune complexes through uptake from the circulation and transcellular transport into the glomeruli for subsequent removal by resident macrophages. f | MHC-I and MHC-II expressing renal IMECs are a target of donor-specific antibodies (DSAs) after kidney transplantation, leading to context-dependent EC activation and altered immunomodulation. g | Renal ECs are phenotypically heterogeneous, owing to their exposure to a heterogeneous microenvironment of differing osmolalities, affecting their inflammatory status. The exact underlying mechanisms and consequences, depending on their anatomical location, require further investigation in vivo. h | The healthy brain is an immune privileged site, and blood–brain barrier (BBB) ECs contribute to this by having tight intercellular junctions and with low or absent expression of adhesion molecules. Upon EC activation in disease (indicated by the red background), the BBB is breached and the brain parenchyma is no longer immune privileged. i | ECs from the aged mouse brain show heterogeneity in (increased) cytokine signalling in arteries, veins and capillaries, possibly increasing immune cell recruitment properties and consequently increasing EC immunomodulatory status and reducing immune privilege. Asterisks indicate recent insights which we considered novel for IMEC biology. HLA, human leukocyte antigen; IFN, interferon; IL, interleukin; mOsm, milliosmoles; PDL1, programmed death ligand 1; TCR, T cell receptor; TGF, transforming growth factor; TLR, Toll-like receptor; Treg cell, regulatory T cell; VCAM1, vascular cell adhesion molecule 1.
Emerging evidence also indicates that immunomodulation by pulmonary ECs may co-determine disease severity and progression in lung cancer. Tumour ECs (TECs) from individuals with untreated, non-metastatic non-small-cell lung cancer of the squamous cell or adenocarcinoma subtype exhibit decreased expression of genes encoding ICAM1, the chemokines CCL2 and CCL18, the cytokine IL-6 and HLA-I/HLA-II (ref.114), suggesting an immunosuppressive environment115. Additionally, TECs of human and mouse lungs show elevated expression of genes encoding FASL, a cell death regulator capable of inducing cell death in cytotoxic T cells12, and of co-inhibitory molecules such as PDL1, further indicating an immunosuppressive role116 (Fig. 2a). Another single-cell study, of human and mouse lung tumours, illustrated a complex immunomodulatory gene signature75. In line with earlier studies, lung capillary TECs expressed lower levels of immunomodulatory genes (involved in antigen presentation and processing) than peritumoural capillary ECs, suggesting that certain TEC subpopulations might become more tolerogenic75. However, tumours had fewer capillaries, which suggests that further research is required to investigate the exact immunomodulatory role of lung capillary TECs75. Furthermore, mice with a deficiency of MHC-II in non-haematopoietic cells had fewer Treg cells in the lung and a lower pulmonary metastasis burden in lung tumour models109, which may suggest that antigen presentation by pulmonary ECs contributes to immune tolerance in lung cancer, although EC-selective knockout approaches are required to confirm this. However, another population of activated PCV lung ECs that was enriched in human non-small-cell lung cancer and mouse lung tumours was shown to upregulate a HEV-like gene signature and ACKR1 expression, suggesting that there may be different populations of TECs that either promote or suppress tumour immunity75. Notably, mass cytometry revealed high surface expression of HLA-DRA on healthy capillary lung ECs, which was comparable to that on immune cells in general. This finding requires further functional validation, but highlights the immunomodulatory potential of these ECs as non-professional APCs75.
The role of lung ECs has also been investigated in various infection models. For example, in a mouse model of Plasmodium berghei-induced malaria, lung ECs were shown to cross-present malaria parasite antigens to CD8+ T cells (this was also shown in vitro) in response to stimulation by IFNγ, which is presumably secreted by CD8+ T cells (and possibly CD4+ T cells and natural killer cells). This process is associated with vascular leakage and lung damage117 (Fig. 2b), indicating that antigen presentation by lung ECs can have detrimental effects. Vascularized lung-on-chip models allow investigation of the role of lung ECs in infections such as COVID-19. These showed that lung ECs underlying epithelial cells can be directly infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and contained viral RNA (however, without signs of active viral replication), and infected ECs exhibited a decreased barrier integrity118. In aged mice, pulmonary capillary ECs have been shown to upregulate various cytokine transcripts (such as Il1b, Tnf and Tgfb1)119, which suggests that capillaries might contribute to lung diseases that are more prevalent in individuals >65 years of age, such as chronic obstructive pulmonary disease and lung cancer120, and possibly might contribute to the severity of COVID-19 (ref.121). Given that aged individuals are more prone to severe COVID-19, it is possible that SARS-CoV-2 infection of ECs in aged individuals might lead to a more pronounced loss of barrier function and increased hyperinflammation in the lung121. On the other hand, SARS-CoV-2 infection of ECs in a human lung-on-a-chip model has also been shown to decrease CD31 expression and thus impair immune cell recruitment to the lung118.
Similarly, in influenza virus infection, ECs may contribute to the cytokine storm that characterizes severe infection122. Viral replication in mouse ECs has been shown for specific influenza virus strains123, and this might impair the barrier function of the lung epithelium. Hence, viral replication in specific subtypes of ECs, such as capillary ECs, might induce viral antigen presentation and contribute to a rapid recall response of intravascular or perivascular memory T cells124. Together, emerging evidence indicates that pulmonary ECs are involved in immune responses, but whether they promote immunity (and potential tissue pathology in infections) or tolerance appears to be contextual and requires further study.
The kidney
Kidney ECs represent a particularly heterogeneous population, where cortical, glomerular and medullary ECs exert distinct functions in the renal vascular bed and are exposed to different microenvironments depending on where they are located alongside the nephron125,126. Glomerular and peritubular ECs have fenestrations and are exposed to different concentrations of uraemic toxins, which are filtered from blood, and different osmolalities, which may affect their phenotype and their responses to vasoregulation by the renin–angiotensin–aldosterone system125. Indeed, in vitro, elevated sodium chloride concentrations increase the expression of VCAM1 and E-selectin in human ECs and promote the transmigration of mononuclear immune cells and monocytes, and in vivo, higher salt concentrations enhance myeloid cell binding to ECs127,128. In agreement with these observations, newly identified subpopulations of cortical and medullary capillary ECs in healthy kidneys of mice express an interferon-regulated gene expression signature, including an upregulation of MHC-II, the functional consequences of which need to be validated129 (Fig. 2g). Interestingly, medullary capillary ECs from dehydrated mice, which are exposed to non-physiologically high osmolalities, lower their transcriptional response to IFNβ129, indicating that different osmolalities may influence inflammatory responses via their effects on kidney ECs.
To date, studies of the immunomodulatory potential of ECs in the kidney have focused mainly on glomerular ECs. Glomeruli are the blood-filtering hubs of the nephron and contain fenestrations, which allows them to be selectively permeable to water, salts and specific macromolecules. Compared with other ECs, glomerular ECs have a particularly thick filamentous glycocalyx that contributes to the regulation of fluid balance, but also prevents interactions with immune cells. Upon activation of glomerular ECs in response to infection or as a consequence of disease, such as lupus nephritis, shedding of the glycocalyx exposes surface molecules on ECs that facilitate the extravasation of immune cells into the glomeruli125,130,131 (Fig. 2d). This can contribute to immune cell-mediated damage of glomeruli when immune cells such as neutrophils infiltrate the glomeruli and release their granules125. Glomerular ECs also participate in immune responses by filtering circulating immune complexes from the blood into the glomeruli via transcellular transport, where these are removed by glomerular macrophages, which can also initiate an inflammatory response if appropriately stimulated4 (Fig. 2e).
Immunomodulation by renal ECs is of particular interest in the context of organ transplantation. Renal microvascular ECs are frequently targets of donor-specific antibodies that bind to HLA molecules expressed by the transplanted kidney, and ECs contribute to alloimmunity by upregulating HLA-II genes after transplantation132,133 (Fig. 2f). A recent study of transplanted human kidneys documented a not further specified subpopulation of donor ECs in the transplanted kidney that showed signs of activation134 (suggesting that it is a target of donor-specific antibody-mediated rejection) and an upregulation of genes involved in phagocytosis134, which may indicate antibody uptake. Also, under stress conditions, renal ECs (subtype to be specified) produce transforming growth factor-β (TGFβ)135 and can secrete large amounts of IL-6 (ref.136). These cytokines can promote the differentiation of naive CD4+ T cells into either immunosuppressive Treg cells (when only TGFβ is present) or pro-inflammatory T helper 17 (TH17) cells (when TGFβ and IL-6 are present)137. As antigens presented by MHC-II molecules on renal ECs can skew CD4+ T cell differentiation towards either Treg cells or TH17 cells138,139,140, the inflammatory context that renal ECs are exposed to might have an impact on kidney transplantation success.
Thus, different renal EC populations appear to exert distinct immunomodulatory functions during homeostasis and inflammation and require further study. Therapeutic strategies targeted at ECs in donor kidneys before transplantation may allow the tweaking of EC-mediated immunomodulation in such a way that alloimmunity is decreased and transplantation success increased. Finally, in Wilms tumours, a cancer affecting the kidneys, renal TECs upregulate ACKR1 transcription141. Whether the potential for immune cell recruitment by ACKR1+ TECs can be exploited by tuning additional TEC populations to acquire ACKR1 expression to stimulate tumoricidal immune cell infiltration might be of interest as anticancer therapy, given the generally immunosuppressive features of TECs.
The brain
In healthy conditions, the brain is poorly infiltrated by immune cells owing to the low expression of adhesion molecules by the specialized capillary and PCV ECs of the blood–brain barrier (BBB)142 and the abundance of tight junctions between these ECs. Brain ECs thus exhibit a larger level of immune anergy and contribute to the maintenance of the immune privileged state of the brain54. Unlike liver and renal ECs, BBB ECs lack fenestrations and form continuous intercellular junctional complexes, limiting paracellular leakage of molecules from the circulation into the brain. Further, BBB ECs not only express low levels of adhesion molecules (such as ICAM1) but also express lower levels of cytokines and chemokines (such as IL-8 and CCL2), regulated in part by astrocyte-derived sonic hedgehog, which, via hedgehog receptors, induces immune quiescence in ECs, impairing immune cell migration143.
However, in models of infection or inflammatory disease, BBB ECs upregulate adhesion molecules (such as E-selectin and P-selectin) and chemokines (such as CXCL1), thereby promoting immune cell infiltration and inflammation in the brain53,144,145 (Fig. 2h). For example, after transmigration, extravasated monocytes differentiate into TH17-polarizing dendritic cells in response to brain EC-derived granulocyte–macrophage colony-stimulating factor (GM-CSF) and TGFβ146, suggesting a tight regulation of immune cells that interact with brain ECs in mouse models. Intriguingly, depression due to chronic stress alters BBB integrity in animal models, allowing the passage of monocytes and IL-6 from the circulation, and raising the question of whether compromised BBB integrity and depression may indeed be linked147. Interestingly, brain ECs have phagocytotic capacity148, and microvascular ECs of the spinal cord can phagocytose myelin debris and recruit macrophages in vivo14, raising the question of whether specialized brain ECs may process antigens and promote brain inflammation in neurological diseases with an inflammatory component. Indeed, even though BBB ECs have low rates of pinocytosis (suggesting that this is not the main route for extracellular antigens to be acquired), they can present antigens on MHC-I and express MHC-II under inflammatory conditions149,150, which may facilitate adaptive immune responses in the brain by promoting T cell activation and potentially allowing antigen-specific T cells to enter the brain.
scRNA-seq analyses of mouse and human brains provided further insights into the regional heterogeneity of ECs in the brain, in particular in the context of ageing and age-related neurodegenerative disease (Fig. 2i). For example, brain ECs from hippocampi of aged mice upregulate the expression of VCAM1 in a vascular bed-specific pattern151. Indeed, venous and arterial VCAM1+ ECs expressed Tnfrsf1a, Il1r1, Il6ra and Il6st (generally considered to be pro-inflammatory), whereas venous VCAM1+ ECs additionally upregulated genes involved in immune cell infiltration, differentiation and antigen presentation (including Tspo, Lrg1 and B2m) and in pathways involved in TNF and NF-κB signalling151. This suggests that venous brain ECs are the most activated, and thus likely the immune cell-recruiting EC population in aged brains.
Another scRNA-seq study reported VCAM1 expression in a mixed mouse EC population (exhibiting arterial and venous features) but found that it was unaltered in brain ECs from aged brains compared with young brains152. However, aged capillary ECs had increased expression of genes involved in VCAM1-mediated immune cell migration152. Moreover, IFNγ response genes were downregulated in aged arterial and venous ECs compared with young controls, TLR-signalling was upregulated in aged arterial and venous–capillary ECs, and interleukin signalling was predominantly upregulated in aged capillary, venous and capillary–venous ECs152, suggesting a large heterogeneity in inflammatory signalling in ECs from different parts of the aged brain vasculature.
Other scRNA-seq studies document that ageing affects immunomodulation by capillary ECs by upregulating pathways involved in immune cell recruitment to the BBB, but also in innate immunity, TGFβ signalling and antigen processing144, or that ECs from aged mouse brains upregulate the expression of Cxcl12 (ref.153) (encoding a chemotactic ligand for CXCR4-expressing cells154) and Cd9 (ref.153) (encoding a surface protein that promotes the adhesion of immune cells to VCAM1 and ICAM1 (ref.155)). In the entorhinal cortex of patients with Alzheimer disease, ECs upregulated genes involved in the regulation of cytokine secretion and inflammation, including HLA-E (encoding a known natural killer cell modulator), MEF2C and NFKBIA156, indicating that ECs from brain regions affected by Alzheimer disease have a stronger inflammatory signature than brain ECs from age-matched healthy controls. These conflicting reports suggest that ECs from aged brains generally display immunological features that are atypical for ECs from non-aged brains, with the activation of specific subpopulations of brain ECs that are likely to promote the recruitment and functional modulation of immune cells. However, it is unclear which subtypes of brain ECs are most affected by ageing.
Conclusion
We have described the immunomodulatory functions of many different subsets of ECs, which we propose to collectively refer to as ‘IMECs’. The findings discussed herein suggest that (1) IMECs in tissues that are infiltrated by immune cells have specific immune cell-recruiting properties, a feature that can be induced by chronic inflammatory stimuli in non-lymphoid tissues; (2) IMECs in the lung and liver not only promote immune homeostasis but also mediate a careful balance between tolerance and inflammation (their role in immunomodulation may be partially determined by their anatomical location); (3) IMECs in the kidney and liver closely interact with resident immune cells, which may allow swift responses to circulating immune complexes; and (4) IMECs of immune privileged tissues such as the healthy brain form a tight and low immunomodulatory barrier to minimize infiltration of the tissue parenchyma. The capacity of IMECs to facilitate immune homeostasis might be more diverse than realized to date, and appears to depend on the specific subpopulation of ECs in a given tissue and their location in the vascular bed, and may change with age and in response to infection and disease.
However, there are a number of important outstanding questions. For example, it remains to be determined whether IMECs in tumours are tolerogenic or immunostimulatory, and whether they can be rendered more immunostimulatory by promoting their antigen-presenting function. If so, how could this be achieved? Does antigen presentation by IMECs in specific (which?) contexts, organs or conditions promote inflammation or tolerance? And when is antigen specificity a prerequisite for efficient immune cell migration157,158,159? Is the repertoire of antigens (presented by semi-professional antigen-presenting ECs) unique or generic compared with that of professional APCs? How important are IMECs as semi-professional APCs, considering their abundance compared with professional APCs? What is the main mechanism of antigen uptake for the different subtypes of IMECs? Does the apical–basolateral polarity of ECs affect antigen uptake from the circulation or tissue parenchyma? A related question is whether apically expressed MHC and adhesion molecules, which are the first molecules to which recruited T cells bind11, facilitate a sufficiently long interaction between the T cell and the IMEC to allow immunomodulation. Another question is whether some of these molecules are redistributed basolaterally and thereby prolong the duration of IMEC–T cell interaction. What is the contribution of IMECs interacting with perivascular immune cells to tissue immune homeostasis? And adding another layer of complexity, what is the relevance of bone marrow-derived endothelial progenitor cells, which might be recruited to replace injured IMECs3,160, and do these acquire tissue-specific immunomodulatory features similar to those of pre-existing IMECs? Do IMECs develop a form of trained immunity, as observed in in vitro experiments with human aortic ECs161,162,163? EC metabolism affects interferon-stimulated gene expression in ECs via effects on gene methylation, raising the question of how EC metabolism regulates IMEC function across tissues164. Are IMECs polarized towards a pro-inflammatory or an anti-inflammatory phenotype in a tissue-specific manner upon priming by specific pathogen-associated molecular patterns? What are the mechanisms of HEV biogenesis in non-lymphoid tissues? And how do HEVs regulate immunity beyond immune cell recruitment?
The observation that subsets of ECs are involved in immune cell recruitment and vascular inflammation is not novel, but the concept that specific subpopulations of ECs are non-haematopoietic partners in an active immune response is an emerging concept, raising the translationally important question of whether the immunomodulatory capacity of IMECs can be targeted for immunotherapeutic purposes.
References
Jackson, D. G. Leucocyte trafficking via the lymphatic Vasculature — mechanisms and consequences. Front. Immunol. 10, 471 (2019).
Jalkanen, S. & Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 20, 566–578 (2020).
Sørensen, K. K. et al. The scavenger endothelial cell: a new player in homeostasis and immunity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R1217–R1230 (2012).
Stamatiades, E. G. et al. Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 166, 991–1003 (2016). This article describes how renal ECs transport immune complexes from the circulation to perivascular macrophages for optimal cooperative immune monitoring.
Andueza, A. et al. Endothelial reprogramming by disturbed flow revealed by single-cell RNA and chromatin accessibility study. Cell Rep. 33, 108491 (2020). This study describes an endothelial immune cell-like type that arises under disturbed flow and expresses markers commonly associated with macrophages.
Boisset, J.-C. et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010).
Lis, R. et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature 545, 439–445 (2017).
Pober, J. S. & Sessa, W. C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 (2007).
Lohse, A. et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 110, 1175–1181 (1996).
Wedgwood, J. F., Hatam, L. & Bonagura, V. R. Effect of interferon-γ and tumor necrosis factor on the expression of class I and class II major histocompatibility molecules by cultured human umbilical vein endothelial cells. Cell. Immunol. 111, 1–9 (1988).
Carman, C. V. & Martinelli, R. T lymphocyte–endothelial interactions: emerging understanding of trafficking and antigen-specific immunity. Front. Immunol. 6, 603 (2015).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Dini, L. et al. Phagocytosis of apoptotic bodies by liver endothelial cells. J. Cell Sci. 108, 967–973 (1995).
Zhou, T. et al. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 22, 421–435 (2019).
Sha, X. et al. Interleukin-35 inhibits endothelial cell activation by suppressing MAPK-AP-1 pathway. J. Biol. Chem. 290, 19307–19318 (2015).
Mai, J. et al. Interleukin-17A promotes aortic endothelial cell activation via transcriptionally and post-translationally activating p38 mitogen-activated protein kinase (MAPK) pathway. J. Biol. Chem. 291, 4939–4954 (2016).
Ager, A. High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front. Immunol. 8, 45 (2017).
Georganaki, M., van Hooren, L. & Dimberg, A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front. Immunol. 9, 3081 (2018).
Dauphinee, S. M. & Karsan, A. Lipopolysaccharide signaling in endothelial cells. Lab. Invest. 86, 9–22 (2006).
Liao, J. K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Invest. 123, 540–541 (2013).
Muller, W. A. Leukocyte-endothelial cell interactions in the inflammatory response. Lab. Invest. 82, 521–534 (2002).
Scott, H., Brandtzaeg, P., Hirschberg, H., Solheim, B. G. & Thorsby, E. Vascular and renal distribution of HLA-DR-like antigens. Tissue Antigens 18, 195–202 (1981).
Hancock, W. W., Kraft, N. & Atkins, R. C. The immunohistochemical demonstration of major histocompatibility antigens in the human kidney using monoclonal antibodies. Pathology 14, 409–414 (1982).
Hart, D. N. et al. Localization of HLA-ABC and DR antigens in human kidney. Transplantation 31, 428–433 (1981).
Hirschberg, H., Bergh, O. J. & Thorsby, E. Antigen-presenting properties of human vascular endothelial cells. J. Exp. Med. 152, 249s–255s (1980).
Vandenberghe, P., Delabie, J., de Boer, M., De Wolf-Peeters, C. & Ceuppens, J. L. In situ expression of B7/BB1 on antigenpresenting cells and activated B cells: an immunohistochemical study. Int. Immunol. 5, 317–321 (1993).
Kreisel, D. et al. Mouse vascular endothelium activates CD8+ T lymphocytes in a B7-dependent fashion. J. Immunol. 169, 6154–6161 (2002).
Walch, J. M. et al. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J. Clin. Invest. 123, 2663–2671 (2013).
Pober, J. S., Merola, J., Liu, R. & Manes, T. D. Antigen presentation by vascular cells. Front. Immunol. 8, 1907 (2017).
Meehan, S. M. et al. Cytotoxicity and apoptosis in human renal allografts: identification, distribution, and quantitation of cells with a cytotoxic granule protein GMP-17 (TIA-1) and cells with fragmented nuclear DNA. Lab. Investig. J. Tech. Methods Pathol. 76, 639–649 (1997).
Colvin, R. B. et al. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J. Am. Soc. Nephrol. JASN 8, 1930–1941 (1997).
Jutte, N. H. P. M. et al. Human heart endothelial-cell-restricted allorecognition. Transplantation 62, 403–406 (1996).
Al-Lamki, R. S., Bradley, J. R. & Pober, J. S. Endothelial cells in allograft rejection. Transplantation 86, 1340–1348 (2008).
Turesson, C. Endothelial expression of MHC class II molecules in autoimmune disease. Curr. Pharm. Des. 10, 129–143 (2003).
Mai, J., Virtue, A., Shen, J., Wang, H. & Yang, X.-F. An evolving new paradigm: endothelial cells – conditional innate immune cells. J. Hematol. Oncol. J. Hematol. Oncol. 6, 61 (2013).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6, 1348–1354 (2000).
Kotamraju, S. et al. Upregulation of immunoproteasomes by nitric oxide: potential antioxidative mechanism in endothelial cells. Free Radic. Biol. Med. 40, 1034–1044 (2006).
Foss, G. S. & Prydz, H. Interferon regulatory factor 1 mediates the interferon-γ induction of the human immunoproteasome subunit multicatalytic endopeptidase complex-like 1. J. Biol. Chem. 274, 35196–35202 (1999).
Murata, S., Takahama, Y., Kasahara, M. & Tanaka, K. The immunoproteasome and thymoproteasome: functions, evolution and human disease. Nat. Immunol. 19, 923–931 (2018).
Givens, C. & Tzima, E. Endothelial mechanosignaling: does one sensor fit all? Antioxid. Redox Signal. 25, 373–388 (2016).
Sheikh, S., Rainger, G. E., Gale, Z., Rahman, M. & Nash, G. B. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-α: a basis for local variations in vascular sensitivity to inflammation. Blood 102, 2828–2834 (2003).
Chiu, J.-J. et al. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-α in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 73–79 (2004).
Walpola, P. L., Gotlieb, A. I., Cybulsky, M. I. & Langille, B. L. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler. Thromb. Vasc. Biol. 15, 2–10 (1995).
Jankowska, K. I. et al. Integrins modulate T cell receptor signaling by constraining actin flow at the immunological synapse. Front. Immunol. 9, 25 (2018).
Finger, E. B. et al. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature 379, 266–269 (1996).
Paschall, C. D., Guilford, W. H. & Lawrence, M. B. Enhancement of L-selectin, but not P-selectin, bond formation frequency by convective flow. Biophys. J. 94, 1034–1045 (2008).
Lawrence, M. B., Kansas, G. S., Kunkel, E. J. & Ley, K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E). J. Cell Biol. 136, 717–727 (1997).
Huang, R. B. & Eniola-Adefeso, O. Shear stress modulation of IL-1β-induced e-selectin expression in human endothelial cells. PLoS ONE 7, e31874 (2012).
Tinoco, R., Otero, D. C., Takahashi, A. & Bradley, L. M. PSGL-1: a new player in the immune checkpoint landscape. Trends Immunol. 38, 323–335 (2017).
Gold, M. C., Napier, R. J. & Lewinsohn, D. M. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol. Rev. 264, 154–166 (2015).
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, eaay5516 (2020).
Shetty, S., Lalor, P. F. & Adams, D. H. Liver sinusoidal endothelial cells — gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 15, 555–567 (2018).
Sonar, S. A. & Lal, G. Blood-brain barrier and its function during inflammation and autoimmunity. J. Leukoc. Biol. 103, 839–853 (2018).
Spadoni, I., Fornasa, G. & Rescigno, M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat. Rev. Immunol. 17, 761–773 (2017).
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature https://doi.org/10.1038/s41586-020-2424-4 (2020).
Randolph, G. J., Ivanov, S., Zinselmeyer, B. H. & Scallan, J. P. The lymphatic system: integral roles in immunity. Annu. Rev. Immunol. 35, 31–52 (2017).
Bogoslowski, A., Butcher, E. C. & Kubes, P. Neutrophils recruited through high endothelial venules of the lymph nodes via PNAd intercept disseminating Staphylococcus aureus. Proc. Natl Acad. Sci. USA 115, 2449–2454 (2018).
Palframan, R. T. et al. Inflammatory chemokine transport and presentation in HEV. J. Exp. Med. 194, 1361–1374 (2001).
Yoneyama, H. et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16, 915–928 (2004).
Uchimura, K. et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat. Immunol. 6, 1105–1113 (2005).
Mitoma, J. et al. Critical functions of N -glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat. Immunol. 8, 409–418 (2007).
Kawashima, H. et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 6, 1096–1104 (2005).
Maas, S. L., Soehnlein, O. & Viola, J. R. Organ-specific mechanisms of transendothelial neutrophil migration in the lung, liver, kidney, and aorta. Front. Immunol. 9, 2739 (2018).
Yadav, R., Larbi, K., Young, R. & Nourshargh, S. Migration of leukocytes through the vessel wall and beyond. Thromb. Haemost. 90, 598–606 (2003).
Proebstl, D. et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med. 209, 1219–1234 (2012).
Woodfin, A. et al. Junctional adhesion molecule-C (JAM-C) regulates polarized neutrophil transendothelial cell migration in vivo. Nat. Immunol. 12, 761–769 (2011).
Aird William, C. Phenotypic heterogeneity of the endothelium. Circ. Res. 100, 158–173 (2007).
Browning, J. L. et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23, 539–550 (2005).
Kang, S. W. et al. Anti-CD137 suppresses tumor growth by blocking reverse signaling by CD137 ligand. Cancer Res. 77, 5989–6000 (2017).
Yaddanapudi, K. et al. Control of tumor-associated macrophage alternative activation by MIF. J. Immunol. 190, 2984–2993 (2013).
Castro, B. A. et al. Macrophage migration inhibitory factor downregulation: a novel mechanism of resistance to anti-angiogenic therapy. Oncogene 36, 3749–3759 (2017).
Li, Z., He, L., Wilson, K. & Roberts, D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J. Immunol. 166, 2427–2436 (2001).
Veerman, K., Tardiveau, C., Martins, F., Coudert, J. & Girard, J.-P. Single-cell analysis reveals heterogeneity of high endothelial venules and different regulation of genes controlling lymphocyte entry to lymph nodes. Cell Rep. 26, 3116–3131.e5 (2019). This work uses scRNA-seq to describe HEV heterogeneity in murine lymph nodes during homeostasis, dedifferentiation and inflammation.
Brulois, K. et al. A molecular map of murine lymph node blood vascular endothelium at single cell resolution. Nat. Commun. 11, 3798 (2020). This scRNA-seq study identifies non-high-vein ECs in murine lymph nodes which are important for myeloid cell migration into inflamed lymph nodes.
Goveia, J. et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell 37, 21–36.e13 (2020). This work describes endothelial heterogeneity at the single-cell level in peritumoural lung tissues and lung tumour, and identifies EC subpopulations with immune cell recruitment, antigen presentation and scavenging capacity.
Qian, J. et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. https://doi.org/10.1038/s41422-020-0355-0 (2020).
Rohlenova, K. et al. Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis. Cell Metab. 31, 862–877.e14 (2020).
Allen, E. et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak9679 (2017).
He, B. et al. Remodeling of metastatic vasculature reduces lung colonization and sensitizes overt metastases to immunotherapy. Cell Rep. 30, 714–724.e5 (2020).
Johansson-Percival, A. et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol. 18, 1207–1217 (2017).
Uhrig, A. et al. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J. Leukoc. Biol. 77, 626–633 (2005).
Wu, J. et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology 129, 363–374 (2010).
Elvevold, K. et al. Liver sinusoidal endothelial cells depend on mannose receptor-mediated recruitment of lysosomal enzymes for normal degradation capacity. Hepatology 48, 2007–2015 (2008).
Malovic, I. et al. The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology 45, 1454–1461 (2007).
Diehl, L. et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 47, 296–305 (2008).
Limmer, A. et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur. J. Immunol. 35, 2970–2981 (2005).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6, 1348–1354 (2000).
Burgdorf, S., Kautz, A., Böhnert, V., Knolle, P. A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Carambia, A. et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J. Hepatol. 61, 594–599 (2014).
Johansson, A. G., Lövdal, T., Magnusson, K., Berg, T. & Skogh, T. Liver cell uptake and degradation of soluble immunoglobulin G immune complexes in vivo and in vitro in rats. Hepatology 24, 169–175 (1996).
Bertolino, P. et al. Early intrahepatic antigen-specific retention of naïve CD8+ T cells is predominantly ICAM-1/LFA-1 dependent in mice. Hepatology 42, 1063–1071 (2005).
John, B. & Crispe, I. N. Passive and active mechanisms trap activated CD8+ T cells in the liver. J. Immunol. 172, 5222–5229 (2004).
Shetty, S. et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J. Immunol. 186, 4147–4155 (2011).
Halpern, K. B. et al. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat. Biotechnol. 36, 962–970 (2018).
MacParland, S. A. et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 1–21 (2018). This study provides a description of LSECs from different hepatic zones with distinct transcriptomes and putative immunomodulatory functions based on scRNA-seq data.
Gola, A. et al. Commensal-driven immune zonation of the liver promotes host defence. Nature https://doi.org/10.1038/s41586-020-2977-2 (2020). This article describes how perivascular immune cells and liver ECs cooperate for optimal host defence.
Yu, X. et al. Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis. Nat. Commun. 10, 1–14 (2019).
Sharma, A. et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell 183, 377–394.e21 (2020). This work uses scRNA-seq to describe an immunosuppressive niche in hepatocellular carcinoma involving subtypes of ECs, macrophages and Treg cells.
Knolle, P. A. et al. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology 24, 824–829 (1996).
Xu, B. et al. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am. J. Pathol. 163, 1275–1289 (2003).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019). The scRNA-seq data in this study allow the identification of a fibrosis-enriched EC subpopulation with distinct immunomodulatory characteristics.
Thiriot, A. et al. Differential DARC/ACKR1 expression distinguishes venular from non-venular endothelial cells in murine tissues. BMC Biol. 15, 45 (2017).
Girbl, T. et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity 49, 1062–1076.e6 (2018).
Nibbs, R. J. B. & Graham, G. J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 13, 815–829 (2013).
Hashimoto, S. et al. Postoperative portal hypertension enhances alloimmune responses after living-donor liver transplantation in patients and in a mouse model. J. Immunol. 203, 1392–1403 (2019).
Almanzar, N. et al. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Mammoto, A. & Mammoto, T. Vascular niche in lung alveolar development, homeostasis, and regeneration. Front. Bioeng. Biotechnol. 7, 318 (2019).
Jambusaria, A. et al. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife 9, e51413 (2020).
Kreisel, D. et al. Cutting edge: MHC class II expression by pulmonary nonhematopoietic cells plays a critical role in controlling local inflammatory responses. J. Immunol. 185, 3809–3813 (2010).
Travaglini, K. J. et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587, 619–625 (2020).
Gillich, A. et al. Capillary cell-type specialization in the alveolus. Nature https://doi.org/10.1038/s41586-020-2822-7 (2020). This study identifies a subtype of alveolar capillaries with transcriptomic features suggesting antigen presentation capacity using scRNA-seq approaches.
Raredon, M. S. B. et al. Single-cell connectomic analysis of adult mammalian lungs. Sci. Adv. 5, eaaw3851 (2019).
Yang, J., Yan, J. & Liu, B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 9, 978 (2018).
Lambrechts, D. et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 24, 1277–1289 (2018).
Klein, D. The tumor vascular endothelium as decision maker in cancer therapy. Front. Oncol. 8, 367 (2018).
Liu, S. et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 11, 1–16 (2020).
Claser, C. et al. Lung endothelial cell antigen cross-presentation to CD8+ T cells drives malaria-associated lung injury. Nat. Commun. 10, 1–16 (2019).
Thacker, V. V. et al. Rapid endothelial infection, endothelialitis and vascular damage characterise SARS-CoV-2 infection in a human lung-on-chip model. EMBO Rep. https://doi.org/10.1101/2020.08.10.243220 (2020).
Angelidis, I. et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 10, 963 (2019).
Meiners, S., Eickelberg, O. & Königshoff, M. Hallmarks of the ageing lung. Eur. Respir. J. 45, 807–827 (2015).
Teuwen, L.-A., Geldhof, V., Pasut, A. & Carmeliet, P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-020-0343-0 (2020).
Teijaro, J. R. et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146, 980–991 (2011).
Tundup, S. et al. Endothelial cell tropism is a determinant of H5N1 pathogenesis in mammalian species. PLoS Pathog. 13, e1006270 (2017).
Anderson, K. G. et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 189, 2702–2706 (2012).
Jourde-Chiche, N. et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 15, 87–108 (2019).
Molema, G. & Aird, W. C. Vascular heterogeneity in the kidney. Semin. Nephrol. 32, 145–155 (2012).
Dmitrieva, N. I. & Burg, M. B. Elevated sodium and dehydration stimulate inflammatory signaling in endothelial cells and promote atherosclerosis. PLoS ONE 10, e0128870 (2015).
Wild, J. et al. Rubbing salt into wounded endothelium: sodium potentiates proatherogenic effects of TNF-α under non-uniform shear stress. Thromb. Haemost. 112, 183–195 (2014).
Dumas, S. J. et al. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J. Am. Soc. Nephrol. 31, 118–138 (2020).
Rabelink, T. J. & de Zeeuw, D. The glycocalyx — linking albuminuria with renal and cardiovascular disease. Nat. Rev. Nephrol. 11, 667–676 (2015).
Satchell, S. The role of the glomerular endothelium in albumin handling. Nat. Rev. Nephrol. 9, 717–725 (2013).
Cross, A. R., Glotz, D. & Mooney, N. The role of the endothelium during antibody-mediated rejection: from victim to accomplice. Front. Immunol. 9, 106 (2018).
Jane-wit, D. et al. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through non-canonical NF-κB signaling in endothelial cells. Circulation 128, 2504–2516 (2013).
Wu, H. et al. Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J. Am. Soc. Nephrol. 29, 2069–2080 (2018).
Pintavorn, P. & Ballermann, B. J. TGF-β and the endothelium during immune injury. Kidney Int. 51, 1401–1412 (1997).
Su, H., Lei, C.-T. & Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front. Immunol. 8, 405 (2017).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector T H 17 and regulatory T cells. Nature 441, 235–238 (2006).
Lion, J. et al. Endothelial cell amplification of regulatory T cells is differentially modified by immunosuppressors and intravenous immunoglobulin. Front. Immunol. https://doi.org/10.3389/fimmu.2017.01761 (2017).
Lion, J. et al. HLA class II antibody activation of endothelial cells promotes Th17 and disrupts regulatory T lymphocyte expansion. Am. J. Transplant. 16, 1408–1420 (2016).
Taflin, C. et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc. Natl Acad. Sci. USA 108, 2891–2896 (2011).
Young, M. D. et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361, 594–599 (2018).
Obermeier, B., Daneman, R. & Ransohoff, R. M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596 (2013).
Alvarez, J. I. et al. The hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731 (2011).
Chen, M. B. et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 30, 4418–4432.e4 (2020).
Majerova, P. et al. Trafficking of immune cells across the blood-brain barrier is modulated by neurofibrillary pathology in tauopathies. PLoS ONE 14, e0217216 (2019).
Ifergan, I. et al. The blood–brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain 131, 785–799 (2008).
Menard, C. et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760 (2017).
Grutzendler, J. et al. Angiophagy prevents early embolus washout but recanalizes microvessels through embolus extravasation. Sci. Transl. Med. 6, 226ra31–226ra31 (2014).
Howland, S. W., Gun, S. Y., Claser, C., Poh, C. M. & Rénia, L. Measuring antigen presentation in mouse brain endothelial cells ex vivo and in vitro. Nat. Protoc. 10, 2016–2026 (2015).
Lopes Pinheiro, M. A. et al. Internalization and presentation of myelin antigens by the brain endothelium guides antigen-specific T cell migration. eLife 5, e13149 (2016).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25, 988–1000 (2019).
Zhao, L. et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat. Commun. 11, 4413 (2020).
Ximerakis, M. et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 22, 1696–1708 (2019).
Janssens, R., Struyf, S. & Proost, P. The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 15, 299–311 (2018).
Brosseau, C., Colas, L., Magnan, A. & Brouard, S. CD9 tetraspanin: a new pathway for the regulation of inflammation? Front. Immunol. 9, 2316 (2018).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019).
Marelli-Berg, F. M. et al. Cognate recognition of the endothelium induces HY-specific CD8+ T-lymphocyte transendothelial migration (diapedesis) in vivo. Blood 103, 3111–3116 (2004).
Marelli-Berg, F. M., Frasca, L., Weng, L., Lombardi, G. & Lechler, R. I. Antigen recognition influences transendothelial migration of CD4+ T cells. J. Immunol. 162, 696–703 (1999).
Fu, H. et al. Self-recognition of the endothelium enables regulatory T-cell trafficking and defines the kinetics of immune regulation. Nat. Commun. 5, 3436 (2014).
Garbuzova-Davis, S., Haller, E., Lin, R. & Borlongan, C. V. Intravenously transplanted human bone marrow endothelial progenitor cells engraft within brain capillaries, preserve mitochondrial morphology, and display pinocytotic activity towards BBB repair in ischemic stroke rats. Stem Cell Dayt. Ohio 35, 1246–1258 (2017).
Lu, Y. et al. Increased acetylation of H3K14 in the genomic regions that encode trained immunity enzymes in lysophosphatidylcholine-activated human aortic endothelial cells – novel qualification markers for chronic disease risk factors and conditional DAMPs. Redox Biol. 24, 101221 (2019).
Li, X. et al. Anti-inflammatory cytokines IL-35 and IL-10 block atherogenic lysophosphatidylcholine-induced, mitochondrial ROS-mediated innate immune activation, but spare innate immune memory signature in endothelial cells. Redox Biol. 28, 101373 (2019).
Drummer, C. et al. Trained immunity and reactivity of macrophages and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 41, 1032–1046 (2020).
Stone, O. A. et al. Loss of pyruvate kinase M2 limits growth and triggers innate immune signaling in endothelial cells. Nat. Commun. 9, 4077 (2018).
Huber, S. A. & Sartini, D. Roles of tumor necrosis factor alpha (TNF-α) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J. Virol. 79, 2659–2665 (2005).
Yang, L., Jhaveri, R., Huang, J., Qi, Y. & Diehl, A. M. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab. Invest. 87, 927–937 (2007).
Pandey, A. K., Brown, J. D., Harrison, D. G. & Itani, H. A. CD70 modulates the role of eNOS in endothelial cells. FASEB J. 32, 845.7–845.7 (2018).
Watts, C., West, M. A. & Zaru, R. TLR signalling regulated antigen presentation in dendritic cells. Curr. Opin. Immunol. 22, 124–130 (2010).
Brutkiewicz, R. R. Cell signaling pathways that regulate antigen presentation. J. Immunol. 197, 2971–2979 (2016).
Zhu, D. et al. Major histocompatibility complexes are up-regulated in glomerular endothelial cells via activation of c-Jun N-terminal kinase in 5/6 nephrectomy mice. Br. J. Pharmacol. 177, 5131–5147 (2020).
Xie, R. et al. Phagocytosis by macrophages and endothelial cells inhibits procoagulant and fibrinolytic activity of acute promyelocytic leukemia cells. Blood 119, 2325–2334 (2012).
Fauvarque, M. O. & Williams, M. J. Drosophila cellular immunity: a story of migration and adhesion. J. Cell Sci. 124, 1373–1382 (2011).
Thomas, G., Tacke, R., Hedrick, C. C. & Hanna, R. N. Nonclassical patrolling monocyte function in the vasculature. Arterioscler. Thromb. Vasc. Biol. 35, 1306–1316 (2015).
Almodovar, C. R. D., Luttun, A. & Carmeliet, P. An SDF-1 trap for myeloid cell stimulates angiogenesis. Cell 124, 18–21 (2006).
Schmidt, T. & Carmeliet, P. Bridges that guide and unite. Nature 465, 697–699 (2010).
Burzyn, D., Benoist, C. & Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 14, 1007–1013 (2013).
Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Jenne, C. N. & Kubes, P. Immune surveillance by the liver. Nat. Immunol. 14, 996–1006 (2013).
Han, X. et al. Construction of a human cell landscape at single-cell level. Nature https://doi.org/10.1038/s41586-020-2157-4 (2020).
Daar, A. S., Fuggle, S. V., Fabre, J. W., Ting, A. & Morris, P. J. The detailed distribution of MHC class II antigens in normal human organs. Transplantation 38, 293–298 (1984).
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Berg, M. et al. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur. J. Immunol. 36, 2960–2970 (2006).
Odobasic, D. et al. Glomerular expression of CD80 and CD86 is required for leukocyte accumulation and injury in crescentic glomerulonephritis. J. Am. Soc. Nephrol. 16, 2012–2022 (2005).
Seino, K. et al. CD86 (B70/B7–2) on endothelial cells co-stimulates allogeneic CD4+ T cells. Int. Immunol. 7, 1331–1337 (1995).
Jollow, K. C., Zimring, J. C., Sundstrom, J. B. & Ansari, A. A. CD40 ligation induced phenotypic and functional expression of CD80 by human cardiac microvascular endothelial cells1. Transplantation 68, 430–439 (1999).
Prat, A., Biernacki, K., Becher, B. & Antel, J. P. B7 expression and antigen presentation by human brain endothelial cells: requirement for proinflammatory cytokines. J. Neuropathol. Exp. Neurol. 59, 129–136 (2000).
Omari, K. I. M. & Dorovini-Zis, K. Expression and function of the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) in an in vitro model of the human blood–brain barrier. J. Neuroimmunol. 113, 129–141 (2001).
Tan, P. H. et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis 173, 171–183 (2004).
Lozanoska-Ochser, B., Klein, N. J., Huang, G. C., Alvarez, R. A. & Peakman, M. Expression of CD86 on human islet endothelial cells facilitates T cell adhesion and migration. J. Immunol. 181, 6109–6116 (2008).
Yao, S. et al. B7-H2 is a costimulatory ligand for CD28 in human. Immunity 34, 729–740 (2011).
Aicher, A. et al. Characterization of human inducible costimulator ligand expression and function. J. Immunol. 164, 4689–4696 (2000).
Khayyamian, S. et al. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc. Natl Acad. Sci. USA 99, 6198–6203 (2002).
Shiao, S. L., McNiff, J. M. & Pober, J. S. Memory T cells and their costimulators in human allograft injury. J. Immunol. 175, 4886–4896 (2005).
Hughes, C. C., Savage, C. O. & Pober, J. S. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J. Exp. Med. 171, 1453–1467 (1990).
Acknowledgements
The authors thank L. de Rooij for assistance in searching for literature on single-cell RNA sequencing analyses of endothelial cells, and M. Dewerchin for critically reviewing the manuscript. J.A. is supported by a Marie Skłodowska-Curie individual fellowship and the Fonds Wetenschappelijk Onderzoek (FWO). The work of P.C. is supported by long-term structural Methusalem funding by the Flemish Government, FWO, the Foundation Against Cancer (2016-078), a European Research Council (ERC) Advanced Research Grant (EU-ERC743074) and an ERC Proof of Concept grant (ERC-713758), VIB TechWatch and a Novo Nordisk Foundation Laureate Research Grant (NNF19OC0055802).
Author information
Authors and Affiliations
Contributions
P.C. pioneered the immunomodulatory endothelial cell concept, and J.A. and P.C. elaborated on this concept. J.A., G.E. and P.C. wrote the manuscript and created the figures.
Corresponding author
Ethics declarations
Competing interests
P.C. declares associations with Montis Biosciences, Leuven, Belgium, of which he was a scientific co-founder. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Ronen Alon, Xiao-Feng Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amersfoort, J., Eelen, G. & Carmeliet, P. Immunomodulation by endothelial cells — partnering up with the immune system?. Nat Rev Immunol 22, 576–588 (2022). https://doi.org/10.1038/s41577-022-00694-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-022-00694-4
This article is cited by
-
Navigating tumor angiogenesis: therapeutic perspectives and myeloid cell regulation mechanism
Angiogenesis (2024)
-
Ink-structing the future of vascular tissue engineering: a review of the physiological bioink design
Bio-Design and Manufacturing (2024)
-
Early microvascular coronary endothelial dysfunction precedes pembrolizumab-induced cardiotoxicity. Preventive role of high dose of atorvastatin
Basic Research in Cardiology (2024)
-
CAFs vs. TECs: when blood feuds fuel cancer progression, dissemination and therapeutic resistance
Cellular Oncology (2024)
-
A single nuclear transcriptomic characterisation of mechanisms responsible for impaired angiogenesis and blood-brain barrier function in Alzheimer’s disease
Nature Communications (2024)