Abstract
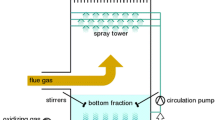
Sulfur dioxide in flue gas from coal-fired power plants presents both ecological and health hazards. Scrubbing dilute, sulfurous flue gas with carbonate eutectic melt at 823K can mitigate these hazards, but for an efficient and economical process, the melt must be regenerated and any resulting contaminants, in particular carbonyl sulfide gas (COS), a known neurotoxin, eliminated. In order to characterize this final stage in the recycling process, the thermal decomposition of COS was studied. A laboratory-scale, quartz flow reactor operating in the temperature range 773–1123 K was selected, with the optional addition of one of three potential catalysts to promote decomposition: an untreated or aqua regia-treated catalytic convertor, γ-alumina powder and charcoal. The extent of decomposition and the reaction products were monitored via gas chromatography as a function of reactor temperature and gas residence time. The feed gas concentration of COS was generally held constant at 30 mol%, the remainder being N2. Under the conditions of our study, 7–44 min residence time, catalytic effects on the kinetics of the COS decomposition reaction were not observed. AddingCO2, thereby lowering the feed COS concentration to 20 mol%, resulted in increased COS decomposition for T < 923 K. Reducing the flow rate from 60 to10 ml/min led to marked increase in the extent of COS thermal decomposition : gas chromatography evaluated COS decomposition at ≈76% following 44 min residence time in the reactor at 823 K. Under no conditions of time or temperature, were reaction products other than CO and sulfur detected.







Similar content being viewed by others
REFERENCES
Kaplan, V., Wachtel, E., Dosmukhamedov, N., and Lubomirsky, I., Int. J. Oil, Gas Coal Technol., 2018, vol. 18, nos. 1–2, p. 25.
Kaplan, V., Wachtel, E., and Lubomirsky, I., RSC Adv., 2013, vol. 3, no. 36, p. 15842.
Yosim, S.J., Grantham, L.F., Mckenzie, D.E., and Stegmann, G.C., Adv. Chem. Ser., 1973, vol. 127, p. 174.
Salem, A., Soliman, A., and El-Haty, I., Air Quality, Atmosph. Health, 2009, vol. 9, nos. 2–3, p. 133.
Lee, J., Cho, H., Moon, I., Lubomirsky, I., Kaplan, V., Kim, J., and Ahn, J., Comput. Chem. Eng., 2021, vol. 146, p. 1.
Nannen, L.W., West, R.E., and Kreith, F., J. Air Pollut. Control Assoc., 1974, vol. 24, no. 1, p. 29.
Davenport, W.G., King, M., Schlesinger, M., and Biswas, A.K., Extractive Metallurgy of Copper, Oxford: Pergamon Press, 2002.
Nolan, P., Coal-Tech 2000 Int. Conf., Jakarta, 2000, p. 1.
Klimont, Z., Smith, S.J., and Cofala, J., Environ. Res. Lett., 2013, vol. 8, p. 1.
Haas, L.A. and Khalafalla, S.E., J. Catal., 1973, vol. 30, p. 451.
Clark, P.D., Dowling, N.I., Huang, M., Svrcek, W.Y., and Monnery, W.D., Ind. Eng. Chem. Res., 2001, vol. 40, no. 2, p. 497.
Partington, J.R. and Neville, H.H., J. Chem. Soc. (London), 1951, p. 1230.
Karan, K., Megrotra, A., and Behie, L., Chem. Eng. Commun., 2005, vol. 192, p. 370.
Sames, J.A. and Paskall, H.G., Sulphur, 1984, vol. 172, p. 47.
Turkdogan, E.T., Physical Chemistry of High Temperature Technology, New York: Academic Press, 1980.
Chase, M.W., NIST-JANAF Thermochemical Tables, New York: Am. Chem. Soc., 1998.
Cardona-Vargas, A., Valencia, D., Arrieta, C.E., and Amell, A., J. Phys.: Conf. Ser., 2020, vol. 1708, p. 1.
Steven, S.C., Chuang, S., and Zhang, L., Handbook of Climate Change Mitigation and Adaptation, New York: Springer, 2015.
ACKNOWLEDGMENTS
The authors thank Dr. Konstantin Gartsman and Dr. Yishay Feldman for their expert assistance in identifying the elemental composition and X-ray diffraction phase, respectively, of the octasulfur crystals deposited in the cooled trap. This work is made possible in part by the historic generosity of the Harold Perlman Family.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence.
About this article
Cite this article
Taichman, O., Kaplan, V., Wachtel, E. et al. Thermal Decomposition of COS to CO and Sulfur: Byproducts of Flue Gas Scrubbing. Solid Fuel Chem. 56, 21–28 (2022). https://doi.org/10.3103/S0361521922010074
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0361521922010074