Abstract
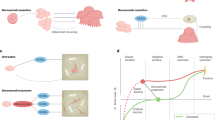
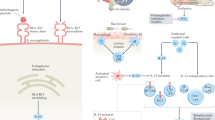
Generalized bone loss (osteoporosis) and fragility fractures can occur in rheumatic and musculoskeletal diseases including rheumatoid arthritis and spondyloarthritis (SpA; including ankylosing spondylitis and psoriatic arthritis). In addition, rheumatoid arthritis can involve localized, periarticular bone erosion and, in SpA, local (pathological) bone formation can occur. The RANK–RANKL–osteoprotegerin axis and the Wnt–β-catenin signalling pathway (along with its inhibitors sclerostin and Dickkopf 1) have been implicated in inflammatory bone loss and formation, respectively. Targeted therapies including biologic DMARDs and Janus kinase (JAK) inhibitors can stabilize bone turnover and inhibit radiographic joint damage, and potentially also prevent generalized bone loss. Targeted therapies interfere at various points in the mechanisms of local and generalized bone changes in systemic rheumatic diseases, and they effect biomarkers of bone resorption and formation, bone mass and risk of fragility fractures. Studies on the effects of targeted therapies on rates of fragility fracture are scarce. The efficacy of biologic DMARDs for arresting bone formation in axial SpA is debated. Improved understanding of the most relevant therapeutic targets and identification of important targeted therapies could lead to the preservation of bone in inflammatory rheumatic and musculoskeletal diseases.
Key points
-
Several molecules, especially inflammatory mediators, contribute to localized bone resorption and formation and to generalized osteoporosis associated with inflammatory rheumatic and musculoskeletal diseases.
-
Targeted therapies could balance the pathological bone turnover in rheumatoid arthritis; however, their effects on bone formation in spondyloarthritis are not equivocal and might depend on the disease stage.
-
Most targeted therapies, particularly TNF inhibitors, might attenuate generalized bone loss in inflammatory rheumatic and musculoskeletal diseases, but more information is needed on the effects of other biologic DMARDs and Janus kinase (JAK) inhibitors.
-
Few studies have assessed the effects of targeted therapies on the risk of fragility fractures; more trials need to be conducted.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lems, W. F. & Dijkmans, B. A. Should we look for osteoporosis in patients with rheumatoid arthritis? Ann. Rheum. Dis. 57, 325–327 (1998).
Walsh, N. C., Crotti, T. N., Goldring, S. R. & Gravallese, E. M. Rheumatic diseases: the effects of inflammation on bone. Immunol. Rev. 208, 228–251 (2005).
Szentpetery, A. et al. Effects of targeted therapies on the bone in arthritides. Autoimmun. Rev. 16, 313–320 (2017).
Raterman, H. G., Bultink, I. E. & Lems, W. F. Osteoporosis in patients with rheumatoid arthritis: an update in epidemiology, pathogenesis, and fracture prevention. Expert Opin. Pharmacother. 21, 1725–1737 (2020).
Raterman, H. G. & Lems, W. F. Pharmacological management of osteoporosis in rheumatoid arthritis patients: a review of the literature and practical guide. Drugs Aging 36, 1061–1072 (2019).
Magrey, M. & Khan, M. A. Osteoporosis in ankylosing spondylitis. Curr. Rheumatol. Rep. 12, 332–336 (2010).
Petho, Z. et al. Characterization of bone metabolism in Hungarian psoriatic arthritis patients: a case-control study. BMC Musculoskelet. Disord. 22, 70 (2021).
Baraliakos, X. & Braun, J. Biologic therapies for spondyloarthritis: what is new? Curr. Rheumatol. Rep. 14, 422–427 (2012).
Lories, R. J., de Vlam, K. & Luyten, F. P. Are current available therapies disease-modifying in spondyloarthritis? Best Pract. Res. Clin. Rheumatol. 24, 625–635 (2010).
Zerbini, C. A. F. et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos. Int. 28, 429–446 (2017).
Ashany, D., Stein, E. M., Goto, R. & Goodman, S. M. The effect of TNF inhibition on bone density and fracture risk and of IL17 inhibition on radiographic progression and bone density in patients with axial spondyloarthritis: a systematic literature review. Curr. Rheumatol. Rep. 21, 20 (2019).
Schett, G. & Gravallese, E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 8, 656–664 (2012).
Lories, R. J. & Haroon, N. Bone formation in axial spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 28, 765–777 (2014).
Boers, N., Michielsens, C. A. J., van der Heijde, D., den Broeder, A. A. & Welsing, P. M. J. The effect of tumour necrosis factor inhibitors on radiographic progression in axial spondyloarthritis: a systematic literature review. Rheumatology 58, 1907–1922 (2019).
Geusens, P. P. et al. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. 54, 1772–1777 (2006).
van den Berg, W. B. & Miossec, P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 549–553 (2009).
Lam, J. et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 106, 1481–1488 (2000).
Sennels, H. et al. Circulating levels of osteopontin, osteoprotegerin, total soluble receptor activator of nuclear factor-kappa B ligand, and high-sensitivity C-reactive protein in patients with active rheumatoid arthritis randomized to etanercept alone or in combination with methotrexate. Scand. J. Rheumatol. 37, 241–247 (2008).
Gulyas, K. et al. Effects of 1-year anti-TNF-alpha therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin. Rheumatol. 39, 167–175 (2020).
Yago, T. et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res. Ther. 9, R96 (2007).
Le Goff, B. et al. Implication of IL-17 in bone loss and structural damage in inflammatory rheumatic diseases. Mediators Inflamm. 2019, 8659302 (2019).
Shah, M. et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 6, e001306 (2020).
Axmann, R. et al. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 60, 2747–2756 (2009).
LaBranche, T. P. et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum. 64, 3531–3542 (2012).
Murakami, K. et al. A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro. PLoS ONE 12, e0181126 (2017).
Adam, S. et al. JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci. Transl Med. 12, eaay4447 (2020).
Gaber, T. et al. Impact of Janus kinase inhibition with tofacitinib on fundamental processes of bone healing. Int. J. Mol. Sci. 21, 865 (2020).
Vidal, B. et al. Effects of tofacitinib in early arthritis-induced bone loss in an adjuvant-induced arthritis rat model. Rheumatology 57, 1461–1471 (2018).
Kleyer, A. et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann. Rheum. Dis. 73, 854–860 (2014).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Sun, M. et al. Anticitrullinated protein antibodies facilitate migration of synovial tissue-derived fibroblasts. Ann. Rheum. Dis. 78, 1621–1631 (2019).
Kleyer, A. & Schett, G. Arthritis and bone loss: a hen and egg story. Curr. Opin. Rheumatol. 26, 80–84 (2014).
Stemmler, F. et al. Biomechanical properties of bone are impaired in patients with ACPA-positive rheumatoid arthritis and associated with the occurrence of fractures. Ann. Rheum. Dis. 77, 973–980 (2018).
Schett, G., Zwerina, J. & David, J. P. The role of Wnt proteins in arthritis. Nat. Clin. Pract. Rheumatol. 4, 473–480 (2008).
Diarra, D. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163 (2007).
Chen, X. X. et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann. Rheum. Dis. 72, 1732–1736 (2013).
Cosman, F. & Dempster, D. W. Anabolic agents for postmenopausal osteoporosis: how do you choose? Curr. Osteoporos. Rep. 19, 189–205 (2021).
Ma, Y. et al. The serum level of Dickkopf-1 in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int. Immunopharmacol. 59, 227–232 (2018).
Fassio, A. et al. In psoriatic arthritis Dkk-1 and PTH are lower than in rheumatoid arthritis and healthy controls. Clin. Rheumatol. 36, 2377–2381 (2017).
Shi, J., Ying, H., Du, J. & Shen, B. Serum sclerostin levels in patients with ankylosing spondylitis and rheumatoid arthritis: a systematic review and meta-analysis. Biomed. Res. Int. 2017, 9295313 (2017).
Appel, H. et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 60, 3257–3262 (2009).
Heiland, G. R. et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann. Rheum. Dis. 69, 2152–2159 (2010).
Yeremenko, N. et al. TNF-alpha and IL-6 differentially regulate Dkk-1 in the inflamed arthritic joint. Arthritis Rheumatol. 67, 865 (2015).
Fassio, A. et al. Acute effects of glucocorticoid treatment, TNFα or IL-6R blockade on bone turnover markers and Wnt inhibitors in early rheumatoid arthritis: a pilot study. Calcif. Tissue Int. 106, 371–377 (2020).
Terpos, E. et al. Early effects of IL-6 receptor inhibition on bone homeostasis: a pilot study in women with rheumatoid arthritis. Clin. Exp. Rheumatol. 29, 921–925 (2011).
Briot, K. et al. The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Joint Bone Spine 82, 109–115 (2015).
Kwon, S. R. et al. Dickkopf-1 level is lower in patients with ankylosing spondylitis than in healthy people and is not influenced by anti-tumor necrosis factor therapy. Rheumatol. Int. 32, 2523–2527 (2012).
Heiland, G. R. et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann. Rheum. Dis. 71, 572–574 (2012).
Saad, C. G. et al. Low sclerostin levels: a predictive marker of persistent inflammation in ankylosing spondylitis during anti-tumor necrosis factor therapy? Arthritis Res. Ther. 14, R216 (2012).
Wu, M. et al. Dickkopf-1 in ankylosing spondylitis: review and meta-analysis. Clin. Chim. Acta 481, 177–183 (2018).
Aschermann, S. et al. Presence of HLA-B27 is associated with changes of serum levels of mediators of the Wnt and hedgehog pathway. Joint Bone Spine 83, 43–46 (2016).
Fassio, A. et al. Secukinumab produces a quick increase in WNT signalling antagonists in patients with psoriatic arthritis. Clin. Exp. Rheumatol. 37, 133–136 (2019).
Barnabe, C. & Hanley, D. A. Effect of tumor necrosis factor alpha inhibition on bone density and turnover markers in patients with rheumatoid arthritis and spondyloarthropathy. Semin. Arthritis Rheum. 39, 116–122 (2009).
Szentpétery, Á., Bhattoa, H. P., Antal-Szalmas, P., Szekanecz, Z. & FitzGerald, O. Circulating mediators of bone remodelling in patients with psoriatic and rheumatoid arthritis treated with anti-TNF-alpha therapy. Arthritis Rheum. 63 (Suppl. 10), S200 (2011).
Vis, M. et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFκB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 65, 1495–1499 (2006).
Visvanathan, S. et al. Effects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitis. Ann. Rheum. Dis. 68, 175–182 (2009).
Vis, M. et al. Early changes in bone metabolism in rheumatoid arthritis patients treated with infliximab. Arthritis Rheum. 48, 2996–2997 (2003).
Woo, J. H., Lee, H. J., Sung, I. H. & Kim, T. H. Changes of clinical response and bone biochemical markers in patients with ankylosing spondylitis taking etanercept. J. Rheumatol. 34, 1753–1759 (2007).
Chopin, F. et al. Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann. Rheum. Dis. 67, 353–357 (2008).
Fassio, A. et al. Inhibition of tumor necrosis factor-alpha (TNF-alpha) in patients with early rheumatoid arthritis results in acute changes of bone modulators. Int. Immunopharmacol. 67, 487–489 (2019).
Garnero, P., Tabassi, N. C. & Voorzanger-Rousselot, N. Circulating dickkopf-1 and radiological progression in patients with early rheumatoid arthritis treated with etanercept. J. Rheumatol. 35, 2313–2315 (2008).
Lim, M. J. et al. Early effects of tumor necrosis factor inhibition on bone homeostasis after soluble tumor necrosis factor receptor use. Korean J. Intern. Med. 29, 807–813 (2014).
Szentpetery, A. et al. Periarticular bone gain at proximal interphalangeal joints and changes in bone turnover markers in response to tumor necrosis factor inhibitors in rheumatoid and psoriatic arthritis. J. Rheumatol. 40, 653–662 (2013).
Garnero, P., Thompson, E., Woodworth, T. & Smolen, J. S. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. 62, 33–43 (2010).
Bay-Jensen, A. C. et al. Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin. Arthritis Rheum. 43, 470–478 (2014).
Wheater, G. et al. Changes in bone density and bone turnover in patients with rheumatoid arthritis treated with rituximab, results from an exploratory, prospective study. PLoS ONE 13, e0201527 (2018).
Boumans, M. J. et al. Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann. Rheum. Dis. 71, 108–113 (2012).
Thudium, C. S. et al. The Janus kinase 1/2 inhibitor baricitinib reduces biomarkers of joint destruction in moderate to severe rheumatoid arthritis. Arthritis Res. Ther. 22, 235 (2020).
Hamar, A. et al. Effects of one-year tofacitinib therapy on bone metabolism in rheumatoid arthritis. Osteoporos. Int. 32, 1621–1629 (2021).
Kaaij, M. H. et al. Anti-IL-17A treatment reduces serum inflammatory, angiogenic and tissue remodeling biomarkers accompanied by less synovial high endothelial venules in peripheral spondyloarthritis. Sci. Rep. 10, 21094 (2020).
Gonzalez-Alvaro, I. et al. Baseline serum RANKL levels may serve to predict remission in rheumatoid arthritis patients treated with TNF antagonists. Ann. Rheum. Dis. 66, 1675–1678 (2007).
Maksymowych, W. P. et al. Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum. 56, 1846–1853 (2007).
Schett, G., Coates, L. C., Ash, Z. R., Finzel, S. & Conaghan, P. G. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res. Ther. 13 (Suppl. 1), S4 (2011).
Wu, D. et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology 59, 3172–3180 (2020).
Emery, P. et al. Baricitinib inhibits structural joint damage progression in patients with rheumatoid arthritis — a comprehensive review. Arthritis Res. Ther. 23, 3 (2021).
van der Linden, M. P. et al. Repair of joint erosions in rheumatoid arthritis: prevalence and patient characteristics in a large inception cohort. Ann. Rheum. Dis. 69, 727–729 (2009).
Lukas, C., van der Heijde, D., Fatenajad, S. & Landewe, R. Repair of erosions occurs almost exclusively in damaged joints without swelling. Ann. Rheum. Dis. 69, 851–855 (2010).
Finzel, S. et al. Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann. Rheum. Dis. 78, 1186–1191 (2019).
Schett, G. et al. Enthesitis: from pathophysiology to treatment. Nat. Rev. Rheumatol. 13, 731–741 (2017).
Zhao, Z. et al. Correlation between magnetic resonance imaging (MRI) findings and the new bone formation factor Dkk-1 in patients with spondyloarthritis. Clin. Rheumatol. 38, 465–475 (2019).
Braun, J. et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum. 48, 1126–1136 (2003).
Baraliakos, X. et al. Long-term effects of secukinumab on MRI findings in relation to clinical efficacy in subjects with active ankylosing spondylitis: an observational study. Ann. Rheum. Dis. 75, 408–412 (2016).
Dougados, M. et al. Evaluation of the change in structural radiographic sacroiliac joint damage after 2 years of etanercept therapy (EMBARK trial) in comparison to a contemporary control cohort (DESIR cohort) in recent onset axial spondyloarthritis. Ann. Rheum. Dis. 77, 221–227 (2018).
Maksymowych, W. P. et al. Modification of structural lesions on MRI of the sacroiliac joints by etanercept in the EMBARK trial: a 12-week randomised placebo-controlled trial in patients with non-radiographic axial spondyloarthritis. Ann. Rheum. Dis. 77, 78–84 (2018).
Molnar, C. et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann. Rheum. Dis. 77, 63–69 (2018).
van der Heijde, D. et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann. Rheum. Dis. 77, 699–705 (2018).
Braun, J. et al. Spinal radiographic progression over 2 years in ankylosing spondylitis patients treated with secukinumab: a historical cohort comparison. Arthritis Res. Ther. 21, 142 (2019).
Deodhar, A. et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 71, 599–611 (2019).
Maksymowych, W. P. et al. Tofacitinib is associated with attainment of the minimally important reduction in axial magnetic resonance imaging inflammation in ankylosing spondylitis patients. Rheumatology 57, 1390–1399 (2018).
Bruijnen, S. T. G. et al. Bone formation in ankylosing spondylitis during anti-tumour necrosis factor therapy imaged by 18F-fluoride positron emission tomography. Rheumatology 57, 631–638 (2018).
Christodoulou-Vafeiadou, E. et al. Ectopic bone formation and systemic bone loss in a transmembrane TNF-driven model of human spondyloarthritis. Arthritis Res. Ther. 22, 232 (2020).
Sambrook, P. Tumour necrosis factor blockade and the risk of osteoporosis: back to the future. Arthritis Res. Ther. 9, 107 (2007).
Marotte, H. et al. A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res. Ther. 9, R61 (2007).
Confavreux, C. B. & Chapurlat, R. D. Systemic bone effects of biologic therapies in rheumatoid arthritis and ankylosing spondylitis. Osteoporos. Int. 22, 1023–1036 (2011).
Hein, G. et al. Influence of rituximab on markers of bone remodeling in patients with rheumatoid arthritis: a prospective open-label pilot study. Rheumatol. Int. 31, 269–272 (2011).
Simon, D. et al. Effect of disease-modifying anti-rheumatic drugs on bone structure and strength in psoriatic arthritis patients. Arthritis Res. Ther. 21, 162 (2019).
Szentpetery, A. et al. Striking difference of periarticular bone density change in early psoriatic arthritis and rheumatoid arthritis following anti-rheumatic treatment as measured by digital X-ray radiogrammetry. Rheumatology 55, 891–896 (2016).
Juhasz, B. et al. Comparison of peripheral quantitative computed tomography forearm bone density versus DXA in rheumatoid arthritis patients and controls. Osteoporos. Int. 28, 1271–1277 (2017).
Neumann, A. et al. Cortical bone loss is an early feature of nonradiographic axial spondyloarthritis. Arthritis Res. Ther. 20, 202 (2018).
Yue, J. et al. Repair of bone erosion in rheumatoid arthritis by denosumab: a high-resolution peripheral quantitative computed tomography study. Arthritis Care Res. 69, 1156–1163 (2017).
Juhász, B. et al. Peripheral quantitative computed tomography in the assessment of bone mineral density in anti-TNF-treated rheumatoid arthritis and ankylosing spondylitis patients. BMC Musculoskelet. Disord. 22, 817 (2021).
Shin, A. et al. Comparative risk of osteoporotic fracture among patients with rheumatoid arthritis receiving TNF inhibitors versus other biologics: a cohort study. Osteoporos. Int. 31, 2131–2139 (2020).
van der Weijden, M. A. et al. Etanercept increases bone mineral density in ankylosing spondylitis, but does not prevent vertebral fractures: results of a prospective observational cohort study. J. Rheumatol. 43, 758–764 (2016).
Acknowledgements
The authors’ work is supported by Hungarian National Scientific Research Fund (OTKA) grant No. K 105073 (to H.P.B. and Z.S.); the TÁMOP-4.2.4.A/2-11-1-2012-0001 National Excellence Program co-financed by the European Union and Hungary (to Z.S.) and the European Union G INOP-2.3.2-15-2016-00050 grant (to Z.S.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made a substantial contribution to discussion of the content, wrote and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks A. Fassio, S. Manske, C. Zerbini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soós, B., Szentpétery, Á., Raterman, H.G. et al. Effects of targeted therapies on bone in rheumatic and musculoskeletal diseases. Nat Rev Rheumatol 18, 249–257 (2022). https://doi.org/10.1038/s41584-022-00764-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00764-w
This article is cited by
-
Exploring the mechanism of Celastrol in the treatment of rheumatoid arthritis based on systems pharmacology and multi-omics
Scientific Reports (2024)
-
Insights and implications of sexual dimorphism in osteoporosis
Bone Research (2024)
-
Potential alleviation of bone mineral density loss with Janus kinase inhibitors in rheumatoid arthritis
Clinical Rheumatology (2024)
-
Transcriptome-wide association study identifies new susceptibility genes and pathways for spondyloarthritis
Journal of Orthopaedic Surgery and Research (2023)
-
Inflammation and gut dysbiosis as drivers of CKD–MBD
Nature Reviews Nephrology (2023)