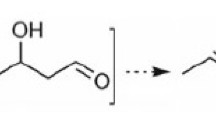
The effect of alkali metal ions (Na+ or K+) on the acid–base and catalytic properties of Zn-Mg(Zr)Si oxide systems in the process of obtaining 1,3-butadiene from ethanol was studied. It was found that the modification of Zn-MgSi oxide systems promotes an increase in the 1,3-butadiene selectivity (at temperatures ≥670 K) by reducing the number of formation sites of by-products. In the composition of Zn-ZrSi oxide systems, alkali metal cation additives lead to a decrease in the ethene and diethyl ether selectivity due to a decrease in the content of strong acid sites that are active in the ethanol dehydration reaction.



Similar content being viewed by others
References
R. Dastillung, B. Fischer, M. Jacquin, and R. Huyghe, “Method for the Production of Butadiene from Ethanol in One Low-Water- and Low-Energy-Consumption Reaction Step”, Patent US 20170267604 A1, Publ. 2017.
G. M. Cabello Gonzalez, A. L. Villanueva Perales, M. Campoy, et al., Fuel Process. Technol., 216, 106767 (2021).
C. E. Cabrera Camacho, B. Alonso-Faricas, A. L. Villanueva Perales, et al., ACS Sustain. Chem. Eng., 8, 10201-10211 (2020).
G. Pomalaza, P. Arango, M. Capron, and F. Dumeignil, Catal. Sci. Technol., 10, 4860-4911 (2020).
P. I. Kyriienko, O. V. Larina, S. O. Soloviev, and S. M. Orlyk, Theor. Exp. Chem., 56, 213-242 (2020).
E. V. Makshina, M. Dusselier, W. Janssens, et al., Chem. Soc. Rev., 43, 7917-7953 (2014).
R. Ohnishi, T. Akimoto, and K. Tanabe, J. Chem. Soc. Chem. Commun., 70, 1613 (1985).
R. A. L. Baylon, J. Sun, and Y. Wang, Catal. Today, 259, 446-452 (2014).
S. Da Ros, M. D. Jones, D. Mattia, et al., ChemCatChem., 8, 2376-2386 (2016).
P. T. Patil, D. Liu, Y. Liu, et al., Appl. Catal. A, 543, 67-74 (2017).
A. Klein, K. Keisers, and R. Palkovits, Appl. Catal. A, 514, 192-202 (2016).
C. Wang and M. Zheng, Green Chem., 21, 1006-1010 (2019).
I. Bin Samsudin, H. Zhang, S. Jaenicke, and G. -K. Chuah, Chem.-Asian J., 15, 4199-4214 (2020).
O. V. Larina, P. I. Kyriienko, and S. O. Soloviev, Theor. Exp. Chem., 51, 252-258 (2015).
V. R. Choudhary, V. H. Rane, and M. Y. Pandit, J. Chem. Technol. Biotechnol., 68, 177-186 (1997).
E. Finazzi, C. Di Valentin, G. Pacchioni, et al., Chem. Eur. J., 14, 4404-4414 (2008).
L. H. Chagas, C. R. V. Matheus, P. C. Zonetti, and L. G. Appel, Mol. Catal., 458, 272-279 (2018).
J. T. Kozlowski and R. J. Davis, J. Energy Chem., 22, 58-64 (2013).
V. V. Ordomsky, V. L. Sushkevich, and I. I. Ivanova, J. Mol. Catal. A, 333, 85-93 (2010).
M. Zhang, Y. Qin, X. Tan, et al., Catal. Lett., 150, 1462-1470 (2020).
Y. Xu, Z. Liu, Z. Han, and M. Zhang, RSC Adv., 7, 7140-7149 (2017).
M. Zhang, X. Tan, T. Zhang, et al., RSC Adv., 8, 34069-34077 (2018).
O. V. Larina, N. D. Shcherban, P. I. Kyriienko, et al., ACS Sustain. Chem. Eng., 8, 16600-16611 (2020).
G. M. Cabello Gonzalez, P. Concepcionb, A. L. Villanueva Peralesa, et al., Fuel Process. Technol., 193, 263-272 (2019).
P. I. Kyriienko, O. V. Larina, D. Y. Balakin, et al., Appl. Catal. A, 616, 118081 (2021).
Acknowledgement
The research was performed with the partial financial support of research programs of the NAS of Ukraine “Support of priority areas of research”, KPKVK 6541230 (0120U101212), “Fundamental problems of creating new substances and materials of chemical production” (0119U101562), and project of research works of young scientists of the National Academy of Sciences of Ukraine (0121U111813).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 57, No. 6, pp. 375-381, November-December, 2021.
Rights and permissions
About this article
Cite this article
Larina, O.V., Kyriienko, P.I., Morozov, O.V. et al. Influence of Modification of Zn-Mg(Zr)Si Oxide Systems by Sodium and Potassium on their Catalytic Properties in the Process of Obtaining 1,3-Butadiene from Ethanol. Theor Exp Chem 57, 443–450 (2022). https://doi.org/10.1007/s11237-022-09714-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-022-09714-9