Abstract
Background
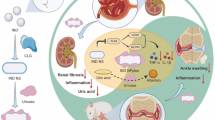
Hyperuricemia is one of the most important risk factors for gout and oxidative stress conditions caused by uric acid excessive production by the xanthine oxidase (XOD). Allopurinol (Allo) is the most widely used inhibitor of XOD, which is used to treat hyperuricemia. Allo not only has a rapid clearance but it also causes some side effects. We prepared a new metal–organic framework (MOF) and compared the release rate and side effects of free Allo and encapsulated in nanocomposite in male Wistar rats.
Method
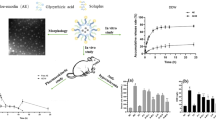
GO/CuFe2O4/IRMOF-3 was prepared by hydrothermal method and Allo was encapsulated in its pores. The structural properties of nanoparticles and efficiency of the free and encapsulated drug in addition to the drug release rate were investigated.
Results
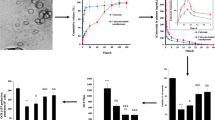
TGA, FTIR, BET, and XRD analyses confirmed the formation of the MOF and the drug encapsulation. SEM images showed dimensions of nanoparticles before/after drug loading were 30 and 50 nm, respectively. Encapsulation caused the slow release of the drug (88% of the loaded drug released in four days). Serum uric acid level in the hyperuricemic group receiving encapsulated Allo was significantly reduced (p < 0.05). The liver and kidney function biomarkers and oxidative stress indices showed a significant improvement. Appearance and histopathology of liver/kidney tissues in all groups were normal.
Conclusion
Encapsulation of Allo in GO/CuFe2O4/IRMOF-3 can be employed to increase the efficacy, reduce the side effects, and lower the release rate of the drugs including treating hyperuricemia and oxidative stress conditions.
Graphical Abstract

















Similar content being viewed by others
References
Maia L, Duarte RO, Ponces-Freire A, Moura JJ, Mira L. NADH oxidase activity of rat and human liver xanthine oxidoreductase: potential role in superoxide production. J Biol Inorg Chem. 2007;12(6):777–87. https://doi.org/10.1007/s00775-007-0229-7.
Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk. Nutr Metab Cardiovasc Dis. 2007;17(6):409–14. https://doi.org/10.1016/j.numecd.2007.02.011.
Bieber JD, Terkeltaub RA. Gout: on the brink of novel therapeutic options for an ancient disease. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2004;50(8):2400–14. https://doi.org/10.1002/art.20438.
Lioté F. Hyperuricemia and gout. Curr Rheumatol Rep. 2003;5(3):227–34. https://doi.org/10.1007/s11926-003-0072-y.
Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Care Res. 2004;51(3):321–5. https://doi.org/10.1002/art.20405.
Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalizations in chronic heart failure: a retrospective cohort study. Heart. 2002;87(3):229–34. https://doi.org/10.1136/heart.87.3.229.
Meng X, Mao Z, Li X, Zhong D, Li M, Jia Y, Zhou H. Baicalein decreases uric acid and prevents hyperuricemic nephropathy in mice. Oncotarget. 2017;8(25):40305. https://doi.org/10.18632/oncotarget.16928.
Lü JM, Yao Q, Chen C. 3,4-Dihydroxy-5-nitrobenzaldehyde (DHNB) is a potent inhibitor of xanthine oxidase: a potential therapeutic agent for treatment of hyperuricemia and gout. Biochem Pharmacol. 2013;86(9):1328–37. https://doi.org/10.1016/j.bcp.2013.08.011.
Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473–80. https://doi.org/10.1038/nrc2394.
Bosetti R, Jones SL. Cost–effectiveness of nanomedicine: estimating the real size of nano-costs. Nanomedicine. 2019;14(11):1367–70. https://doi.org/10.2217/nnm-2019-0130.
Chu C, Su M, Zhu J, Li D, Cheng H, Chen X, Liu G. Metal Organic Framework Nano-particle-Based Biomineralization: A New Strategy toward Cancer Treatment. Theranostics. 2019;9(11):3134–49. https://doi.org/10.7150/thno.33539.
Keskin S, Kızılel S. Biomedical applications of metal organic frameworks. Ind Eng Chem Res. 2011;50(4):1799–812. https://doi.org/10.1021/ie101312k.
Sun Y, Zheng L, Yang Y, Qian X, Fu T, Li X, Yang Z, He Y. Metal–Organic Framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020;12:103. https://doi.org/10.1007/s40820-020-00423-3.
Xia H, Li N, Zhong X, Jiang Y. Metal-organic frameworks: a potential platform for enzyme immobilization and related applications. Front Bioeng Biotechnol. 2020;8:695. https://doi.org/10.3389/fbioe.2020.00695.
Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135(3):372–6. https://doi.org/10.1016/0002-9378(79)90708-7.
Nouri F, Rostamizadeh S, Azad M. Post-synthetic modification of IRMOF-3 with an iminopalladacycle complex and its application as an effective heterogeneous catalyst in Suzuki-Miyaura cross-coupling reaction in H2O/EtOH media at room temperature. Mol Catal. 2017;443:286–93. https://doi.org/10.1016/j.mcat.2017.10.019.
Jimaa RB, Mahmoud ZH, Ali FK. Evaluation the efficiency of CuFe2O4 prepared photolysis by OSD and photo degradation. Entomol Appl Sci Lett. 2018;5(2):91–100.
Deng L, Li Y, Feng F, Wu D, Zhang H. Encapsulation of allopurinol by glucose cross-linked gelatin/zein nano-fibers: Characterization and release behavior. Food Hydrocoll. 2019;94:574–84. https://doi.org/10.1016/j.foodhyd.2019.04.004.
Ullah S, Bustam MA, Assiri MA, Al-Sehemi AG, Kareem FAA, Mukhtar A, Gonfa G. Synthesis and characterization of iso-reticular metal organic framework-3 (IRMOF-3) for CO2/CH4 adsorption: impact of post-synthetic aminomethyl propanol (AMP) functionalization. J Nat Gas Sci Eng. 2019;72:103014. https://doi.org/10.1016/j.jngse.2019.103014.
Wang X, Dou W. Preparation of graphite oxide (GO) and the thermal stability of silicone rubber/GO nano-composites. Thermochim Acta. 2012;529:25–8. https://doi.org/10.1016/j.tca.2011.11.016.
Miatello R, Vzquez M, Renna N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. AJH. 2005;18:864–70. https://doi.org/10.1016/j.amjhyper.2004.12.012.
Lorz DC, Hitchings GH. Specificity of xanthine oxidase. In: Abstracts of the 129th Meeting of the American Chemical Society, Dallas. 1956, p. 30.
Elion GB, Taylor TJ, Hitchings GH. Binding of substrates and inhibitors to xanthine oxidases from different species. VI International Congress of Biochemistry, New York, 1964;42:IV.
Elion GB. Enzymatic and metabolic studies with allopurinol. Ann Rheum Dis. 1966;25(6):608–19. https://doi.org/10.1136/ard.25.suppl_6.608.
Rundles RW, Wyngaarden JB, Hitchings GH, Elion GB, Silberman HR. Effects of a xanthine oxidase inhibitor on thiopurine metabolism, hyperuricemia and gout. Trans Ass Amer Phycns. 1963;76:126–40. https://doi.org/10.3109/9780203214237-143.
Hitchings GH. Effects of allopurinol in relation to purine biosynthesis. Ann Rheum Dis. 1966;25(6):601–10. https://doi.org/10.1136/ard.25.suppl_6.601.
Feigelson P, Davidson JD. The Metabolism of Pyrazolo (3, 4-d) pyrimidines by the Rat. Can Res. 1958;18(2):226–8.
Elion GB, Kovensky A, Hitchings GH. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966;15(7):863–80. https://doi.org/10.1016/0006-2952(66)90163-8.
Elion GB, Kovensky A, Hitchings GH, Metz E, Rundles RW. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966;15:863–8. https://doi.org/10.1016/0006-2952(66)90163.
Aganyants HA, Nikohosyan G, Danielyan KE. Albumin microparticles as the carriers for allopurinol and applicable for the treatment of ischemic stroke. International Nano Letters. 2016;6(1):35–40. https://doi.org/10.1007/s40089-015-0169-0.
Kandav G, Bhatt DC, Jindal DK. Formulation and evaluation of allopurinol loaded chitosan nano-particles. Int J Appl Pharm. 2019;11(3):49–52. https://doi.org/10.22159/ijap.2019v11i3.31932.
Kumar R, Rauti R, Scaini D, Antman-Passig M, Meshulam O, Naveh D, Ballerini L, Shefi O. Graphene-based nano-materials for neuroengineering: recent advances and future prospective. Adv Funct Mater. 2021;2104887. https://doi.org/10.1002/adfm.202104887.
Esfahanian M, Ghasemzadeh MH, Razavian SMH. Synthesis, identification and application of the novel metal-organic framework Fe3O4@PAA@ZIF-8 for the drug delivery of ciprofloxacin and investigation of antibacterial activity. Artif Cells Nanomed Biotechnol. 2019;47(1):2024–30. https://doi.org/10.1080/21691401.2019.1617729.
Miri B, Motakef-Kazemi N, Shojaosadati SA, Morsali A. Application of a nano-porous metal organic framework based on iron carboxylate as drug delivery system. Iranian J Pharm Res: IJPR. 2018;17(4):1164–72 PMCID: PMC6269564. PMID: 30568676.
Cai M, Chen G, Qin L, Qu C, Dong X, Ni J, Yin X. Metal organic frameworks as drug targeting delivery vehicles in the treatment of cancer. Pharmaceutics. 2020;12(3):232–41. https://doi.org/10.3390/pharmaceutics12030232.
Khodabandehloo H, Zahednasab H, Ashrafi HA. Nano-carriers Usage for Drug Delivery in Cancer Therapy. Iran J Cancer Prev. 2016;9(2):3966. https://doi.org/10.17795/ijcp-3966.
Sanaei Rad S, Ghasemzadeh MH, RazavianS MH. Synthesis of a novel ternary ZIF-8/GO/MgFe2O4 nano composite and its application in drug delivery. Sci Rep. 2021;11:18734. https://doi.org/10.1038/s41598-021-98133-2.
de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012;4(1):1–7. https://doi.org/10.1186/1758-5996-4-12.
Fabbrini E, Serafini M, Baric IC, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63(3):976–81. https://doi.org/10.2337/db13-1396.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Accepted.
Consent to Participate
Accepted.
Consent for Publication
Accepted.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mozaffari, F., Razavian, S.M.H. & Ghasemzadeh, M.A. Encapsulation of Allopurinol in GO/CuFe2O4/IR MOF-3 Nanocomposite and In Vivo Evaluation of Its Efficiency. J Pharm Innov 18, 149–163 (2023). https://doi.org/10.1007/s12247-022-09624-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09624-2