Abstract
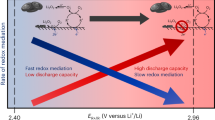
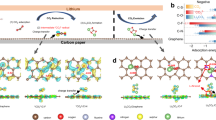
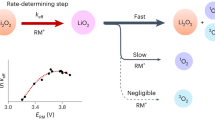
Redox mediators could catalyse otherwise slow and energy-inefficient cycling of Li–S and Li–O2 batteries by shuttling electrons or holes between the electrode and the solid insulating storage materials. For mediators to work efficiently they need to oxidize the solid with fast kinetics but with the lowest possible overpotential. However, the dependence of kinetics and overpotential is unclear, which hinders informed improvement. Here, we find that when the redox potentials of mediators are tuned via, for example, Li+ concentration in the electrolyte, they exhibit distinct threshold potentials, where the kinetics accelerate several-fold within a range as small as 10 mV. This phenomenon is independent of types of mediator and electrolyte. The acceleration originates from the overpotentials required to activate fast Li+/e− extraction and the following chemical step at specific abundant surface facets. Efficient redox catalysis at insulating solids therefore requires careful consideration of the surface conditions of the storage materials and electrolyte-dependent redox potentials, which may be tuned by salt concentrations or solvents.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Qiao, Y., Jiang, K., Deng, H. & Zhou, H. A high-energy-density and long-life lithium-ion battery via reversible oxide–peroxide conversion. Nat. Catal. 2, 1035–1044 (2019).
Cameron, J. M. et al. Molecular redox species for next-generation batteries. Chem. Soc. Rev. 50, 5863–5883 (2021).
Kwak, W.-J. et al. Lithium–oxygen batteries and related systems: potential, status, and future. Chem. Rev. 120, 6626–6683 (2020).
Ko, Y. et al. Anchored mediator enabling shuttle-free redox mediation in lithium-oxygen batteries. Angew. Chem. Int. Ed. 59, 5376–5380 (2020).
Wang, Y. & Lu, Y.-C. Nonaqueous lithium–oxygen batteries: reaction mechanism and critical open questions. Energy Storage Mater. 28, 235–246 (2020).
Wu, S. et al. Organic hydrogen peroxide-driven low charge potentials for high-performance lithium-oxygen batteries with carbon cathodes. Nat. Commun. 8, 15607 (2017).
Qiao, Y. et al. Li-CO2 electrochemistry: a new strategy for CO2 fixation and energy storage. Joule 1, 359–370 (2017).
Lu, Y.-C., He, Q. & Gasteiger, H. A. Probing the lithium–sulfur redox reactions: a rotating-ring disk electrode study. J. Phys. Chem. C. 118, 5733–5741 (2014).
Bonnick, P. & Muldoon, J. The Dr Jekyll and Mr Hyde of lithium sulfur batteries. Energy Environ. Sci. 13, 4808–4833 (2020).
Kwak, W.-J. et al. Controllable and stable organometallic redox mediators for lithium oxygen batteries. Mater. Horiz. 7, 214–222 (2020).
Park, J.-B. et al. Redox mediators for Li–O2 batteries: status and perspectives. Adv. Mat. 30, 1704162 (2018).
Kwak, W.-J. et al. Review—a comparative evaluation of redox mediators for Li-O2 batteries: a critical review. J. Electrochem. Soc. 165, A2274–A2293 (2018).
Meini, S. et al. The use of redox mediators for enhancing utilization of Li2S cathodes for advanced Li–S battery systems. J. Phys. Chem. Lett. 5, 915–918 (2014).
Pande, V. & Viswanathan, V. Criteria and considerations for the selection of redox mediators in nonaqueous Li–O2 batteries. ACS Energy Lett. 2, 60–63 (2017).
McCloskey, B. D. et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 3, 997–1001 (2012).
Wang, Y., Lu, Y.-R., Dong, C.-L. & Lu, Y.-C. Critical factors controlling superoxide reactions in lithium–oxygen batteries. ACS Energy Lett. 5, 1355–1363 (2020).
Liang, Z., Zhou, Y. & Lu, Y.-C. Dynamic oxygen shield eliminates cathode degradation in lithium–oxygen batteries. Energy Environ. Sci. 11, 3500–3510 (2018).
Gao, X. et al. A rechargeable lithium–oxygen battery with dual mediators stabilizing the carbon cathode. Nat. Energy 2, 17118 (2017).
Petit, Y. K. et al. Mechanism of mediated alkali peroxide oxidation and triplet versus singlet oxygen formation. Nat. Chem. 13, 465–471 (2021).
Liang, Z. & Lu, Y.-C. Critical role of redox mediator in suppressing charging instabilities of lithium–oxygen batteries. J. Am. Chem. Soc. 138, 7574–7583 (2016).
Kwak, W.-J. et al. Deactivation of redox mediators in lithium-oxygen batteries by singlet oxygen. Nat. Commun. 10, 1380 (2019).
Liang, Z., Zou, Q., Xie, J. & Lu, Y.-C. Suppressing singlet oxygen generation in lithium–oxygen batteries with redox mediators. Energy Environ. Sci. 13, 2870–2877 (2020).
Mu, X., Pan, H., He, P. & Zhou, H. Li–CO2 and Na–CO2 batteries: toward greener and sustainable electrical energy storage. Adv. Mat. 32, 1903790 (2020).
Zhao, M. et al. An organodiselenide comediator to facilitate sulfur redox kinetics in lithium-sulfur batteries. Adv. Mat. 33, 2007298 (2021).
Zhao, M. et al. Redox comediation with organopolysulfides in working lithium-sulfur batteries. Chem. 6, 3297–3311 (2020).
Tsao, Y. et al. Designing a quinone-based redox mediator to facilitate Li2S oxidation in Li-S batteries. Joule 3, 872–884 (2019).
Chen, Y., Gao, X., Johnson, L. R. & Bruce, P. G. Kinetics of lithium peroxide oxidation by redox mediators and consequences for the lithium–oxygen cell. Nat. Commun. 9, 767 (2018).
Henstridge, M. C., Laborda, E., Rees, N. V. & Compton, R. G. Marcus–Hush–Chidsey theory of electron transfer applied to voltammetry: a review. Electrochim. Acta 84, 12–20 (2012).
Wild, M. et al. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 8, 3477–3494 (2015).
Wang, Y. et al. A solvent-controlled oxidation mechanism of Li2O2 in lithium–oxygen batteries. Joule 2, 2364–2380 (2018).
Zou, Q. & Lu, Y.-C. Solvent-dictated lithium sulfur redox reactions: an operando UV–vis spectroscopic study. J. Phys. Chem. Lett. 7, 1518–1525 (2016).
Ko, Y. et al. A comparative kinetic study of redox mediators for high-power lithium–oxygen batteries. J. Mat. Chem. A. 7, 6491–6498 (2019).
Bawol, P. P. et al. A new thin layer cell for battery related DEMS-experiments: the activity of redox mediators in the Li–O2 cell. Phys. Chem. Chem. Phys. 20, 21447–21456 (2018).
Leverick, G. et al. Solvent-dependent oxidizing power of LiI redox couples for Li–O2 batteries. Joule 3, 1106–1126 (2019).
Zhang, Y. et al. Amorphous Li2O2: chemical synthesis and electrochemical properties. Angew. Chem. Int. Ed. 55, 10717–10721 (2016).
Kang, S., Mo, Y., Ong, S. P. & Ceder, G. A facile mechanism for recharging Li2O2 in Li–O2 batteries. Chem. Mat. 25, 3328–3336 (2013).
Hummelshoj, J. S., Luntz, A. C. & Norskov, J. K. Theoretical evidence for low kinetic overpotentials in Li-O2 electrochemistry. J. Chem. Phys. 138, 034703 (2013).
Zhu, J. et al. Unraveling the catalytic mechanism of Co3O4 for the oxygen evolution reaction in a Li–O2 battery. ACS Catal. 5, 73–81 (2015).
Zhu, J. et al. Surface acidity as descriptor of catalytic activity for oxygen evolution reaction in Li-O2 battery. J. Am. Chem. Soc. 137, 13572–13579 (2015).
Mourad, E. et al. Singlet oxygen from cation driven superoxide disproportionation and consequences for aprotic metal–O2 batteries. Energy Environ. Sci. 12, 2559–2568 (2019).
Mahne, N. et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium–oxygen batteries. Nat. Energy 2, 17036 (2017).
Dai, W. et al. Defect chemistry in discharge products of Li–O2 batteries. Small Methods 3, 1800358 (2019).
Marcus, R. A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 65, 599–610 (1993).
Savéant, J.-M. & Constentin, C. Elements of Molecular and Biomolecular Electrochemistry 2nd edn (John Wiley & Sons, 2019).
Burke, C. M. et al. Implications of 4 e− oxygen reduction via iodide redox mediation in Li–O2 batteries. ACS Energy Lett. 1, 747–756 (2016).
Cornut, R., Griveau, S. & Lefrou, C. Accuracy study on fitting procedure of kinetics SECM feedback experiments. J. Electroanal. Chem. 650, 55–61 (2010).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B. 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B. 13, 5188–5192 (1976).
Methfessel, M. & Paxton, A. T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B. 40, 3616–3621 (1989).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
He, B. et al. High-throughput screening platform for solid electrolytes combining hierarchical ion-transport prediction algorithms. Sci. Data 7, 151 (2020).
Chase, M. W. NIST-JANAF Thermochemical Tables 4th edn, 1510 (ACS, AIP NIST, 1998).
Mo, Y., Ong, S. P. & Ceder, G. First-principles study of the oxygen evolution reaction of lithium peroxide in the lithium-air battery. Phys. Rev. B. 84, 205446 (2011).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant nos. 51773092, 21975124, 11874254, 51802187 and U2030206). It was further supported by Fujian science & technology innovation laboratory for energy devices of China (21C-LAB), Key Research Project of Zhejiang Laboratory (grant no. 2021PE0AC02) and the Cultivation Program for the Excellent Doctoral Dissertation of Nanjing Tech University. S.A.F. is indebted to IST Austria for support.
Author information
Authors and Affiliations
Contributions
Y.C., S.S. and S.A.F. conceived and directed the project. D.C., X.S., A.W., F.Y., and Y.W. performed experiments and DFT calculations. S.A.F., Y.C. and S.S. wrote the paper. All authors discussed and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14, Notes 1–4 and References.
Supplementary Data 1
Atomic coordinates of the optimized computational models.
Source data
Source Data Fig. 1
Source Data Fig. 1.
Source Data Fig. 2
Source Data Fig. 2.
Source Data Fig. 3
Source Data Fig. 3.
Source Data Fig. 4
Source Data Fig. 4.
Source Data Fig. 5
Source Data Fig. 5.
Rights and permissions
About this article
Cite this article
Cao, D., Shen, X., Wang, A. et al. Threshold potentials for fast kinetics during mediated redox catalysis of insulators in Li–O2 and Li–S batteries. Nat Catal 5, 193–201 (2022). https://doi.org/10.1038/s41929-022-00752-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00752-z
This article is cited by
-
Analytical noncovalent electrochemistry for battery engineering
Nature Chemical Engineering (2024)
-
Boosting a practical Li-CO2 battery through dimerization reaction based on solid redox mediator
Nature Communications (2024)
-
Kinetics of redox-mediated catalysis in batteries
Nature Catalysis (2022)
-
Electrochemical Proton Storage: From Fundamental Understanding to Materials to Devices
Nano-Micro Letters (2022)