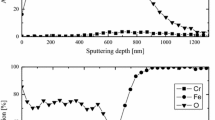
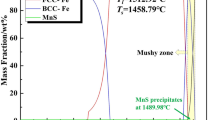
The methods of scanning electron microscopy, energy dispersive analysis and x-ray diffractometry are used to study the kinetics and the mechanism of formation of complex-composition oxide scale on the surface of heat-resistant steel 25Cr18Ni9Si2 during oxidation at 900°C for 500 h. The composition of the scale and the proportion of the gain in the weight to the duration of the oxidation are determined. It is shown that the formation of the composite scale is connected with simultaneous oxidation and reduction of Si, Cr, Fe, and Ni accompanied by refinement of the oxide grains. It is inferred that the dense composite oxide scale is responsible for the high resistance of the steel to oxidation.





Similar content being viewed by others
References
S. Yu. Kondrat’ev, S. N. Petrov, G. P. Anastasiadi, and A. V. Tsemenko, “Structural features of cast refractory alloy HP40NbTi high-temperature oxidation. Part I. Evolution of microstructure and phase composition,” Met. Sci. Heat Treat., 62, 35 – 45 (2020).
S. Yu. Kondrat’ev, S. N. Petrov, G. P. Anastasiadi, and A. V. Tsemenko, “Structural features of cast refractory alloy HP40NbTi high-temperature oxidation. Part 2. Microstructure and phase composition evolution,” Met. Sci. Heat Treat., 62, 46 – 54 (2020).
Liu-zhen Bian, Zhi-yuan Chen, Li-jun Wang, et al., “Oxidation resistance, thermal expansion and area specific resistance of Fe – Cr alloy interconnector for solid oxide fuel cell,” J. Iron Steel Res. Int., 24, 77 – 83 (2017).
C. Y. Cui, C. D. Xia, X. G. Cui, et al., “Novel morphologies and growth mechanism of Cr2O3 oxide formed on stainless steel surface via Nd: YAG pulsed laser oxidation,” J. Alloys Compd., 635, 101 – 106 (2015).
Hongsheng Chen, HaiboWang, Qingzhu Sun, et al., “Oxidation behavior of Fe – 20Cr – 25Ni – Nb austenitic stainless steel in high-temperature environment with small amount of water vapor,” Corros. Sci., 145, 90 – 99 (2018).
Ya-Xin Xu, Xiao-Tao Luo, Cheng-Xin Li, et al., “Formation of Cr2O3 diffusion barrier between Cr-contained stainless steel and cold-sprayed Ni coatings at high temperature,” J. Therm. Spray Technol., 25, 526 – 534(2016).
Mikako Takeda, Hitoshi Kushida, Takashi Onishi, et al., “Influence of oxidation temperature and Cr content on the adhesion and microstructure of scale on low Cr steels,” Oxid. Met., 73, 1 – 13 (2010).
L. C. An, J. Cao, T. Zhang, and Y. T. Yang, “Cr diffusion and continuous repairing behavior during high-temperature oxidation of duplex stainless steel,” Mater. Corros., 68, 1116 – 1122 (2017).
Shoichi Matsubara, Tomiko Yamaguchi, and Fujimitsu Masuyama, “Effect of Cr content and microstructure on high temperature oxidation behavior of high nitrogen heat-resistant ferritic steels,” ISIJ Int., 58, 2102 – 2109 (2018).
Thuan Dinh Nguyen, Jianqiang Zhang, and David J. Young, “Water vapor effects on corrosion of Fe – Cr and Fe – Cr – Ni alloys containing silicon in CO2 gas at 818°C,” Oxid. Met., 83, 575 – 594 (2015).
Hong-huan Mao, Xing Qi, Jing Cao, et al., “Effect of Si on high temperature oxidation of 30Cr13 stainless steel,” J. Iron Steel Res. Int., 24, 561 – 568 (2017).
Xiao-jiang Liu, Yong-quan He, Guang-ming Cao, et al., “Effect of Si content and temperature on oxidation resistance of Fe – Si alloys,” J. Iron Steel Res. Int., 22, 238 – 244 (2015).
L. A. Pisarevskii, G. A. Filippov, and A. A. Lipatov, “Effect of N, Mo, and Si on local corrosion resistance of unstabilized Cr – Ni and Cr – Mn – Ni austenitic steels,” Metallurgist, 60, 822 – 831 (2016).
Jae-Hwang Ko and Dong-Bok Lee, “Oxidation of Ni films electroplated on steel,” Met. Mater. Int., 11, 85 – 88 (2005).
Yi-zhi Liu, Cai-fu Yang, Feng Chai, et al., “High temperature oxidation resistance of 9Ni steel,” J. Iron Steel Res. Int., 21, 956 – 963 (2014).
Dong-liang Li, Gui-qin Fu, Miao-yong Zhu, et al., “Effect of Ni on the corrosion resistance of bridge steel in a simulated hot and humid coastal-industrial atmosphere,” Int. J. Miner. Metall. Mater., 25, 325 – 338 (2018).
T. Jonsson, H. Larsson, S. Karlsson, et al., “High-temperature oxidation of FeCr(Ni) alloys: The behaviour after breakaway,” Oxid. Met., 87, 333 – 341 (2017).
Qing Yuan, Guang Xu,Wei-cheng Liang, et al., “Effects of oxygen content on the oxidation process of Si-containing steel during anisothermal heating,” Int. J. Miner. Metall. Mater., 25, 164 – 172 (2018).
Hong Dong, Pei Wang, Dianzhong Li, and Yiyi Li, “Effect of pre-deformation on the oxidation resistance of a high Si ferritic/martensitic steel in oxygen-saturated stagnant lead-bismuth eutectic at 550°C,” Corros. Sci., 118, 129 – 142 (2017).
Qing Yuan, Guang Xu, Weicheng Liang, et al., “Effects of oxygen concentration on the passivation of Si-containing steel during high-temperature oxidation,” Corros. Rev., 36, 385 – 393 (2018).
Dening Zou, Yuqing Zhou, Xin Zhang, et al., “High temperature oxidation behavior of a high Al-containing ferritic heat-resistant stainless steel,” Mater. Charact., 136, 435 – 443 (2018).
Jian Ren, Liming Yu, Yongchang Liu, et al., “Corrosion behavior of an Al added high-Cr ODS steel in supercritical water at 600°C,” Appl. Surf. Sci., 480, 969 – 978 (2019).
Philippe Refait, Marc Jeannin, Thomas Urios, et al., “Corrosion of low alloy steel in stagnant artificial or stirred natural seawater: The role of Al and Cr,” Mater. Corros., 70, 985 – 995 (2019).
S. Mahboubi, Y. Jiao, W. Cook, et al., “Stability of chromia (Cr2O3)-based scales formed during corrosion of austenitic Fe – Cr – Ni alloys in flowing oxygenated supercritical water,” Corrosion, 72, 1170 – 1180 (2016).
Grzegorz Smola, Richard Gawel, Karol Kyziol, et al., “Influence of nickel on the oxidation resistance at high temperatures of thin chromium coatings,” Oxid. Met., 91, 625 – 640 (2019).
Shoichi Matsubara, Tomiko Yamaguchi, and Fujimitsu Masuyama, “High temperature oxidation behavior of high nitrogen 9% Cr steels,” ISIJ Int., 58, 2095 – 2101 (2018).
S. Yu. Kondrat’ev, G. P. Anastasiadi, A. V. Ptashnik, S. N. Petrov, “The mechanisms of scale and subsurface diffusion zone formation of heat-resistant HP40NbTi alloy at long-term high-temperature exposure,” Materialia, 7, 100427 (2019).
S. Yu. Kondrat’ev, G. P. Anastasiadi, A. V. Ptashnik, S. N. Petrov, “Kinetics of the high-temperature oxidation of heat-resistant statically and centrifugally cast HP40NbTi alloys,” Oxid. Met., 91, 33 – 53 (2019).
G. P. Anastasiadi, S. Yu Kondrat’ev, and A. I. Rudskoy, “Selective high-temperature oxidation of phases in a cast refractory alloy of the 25Cr – 35Ni – Si – Nb – C system,” Met. Sci. Heat Treat., 56, 403 – 408 (2014).
S. Yu Kondrat’ev, G. P. Anastasiadi, A. V. Ptashnik, and S. N. Petrov, “Evolution of the microstructure and phase composition of a subsurface of cast HP-type alloy during a long-term high-temperature aging,” Mater. Charact., 150, 166 – 173 (2019).
S. Yu Kondrat’ev, G. P. Anastasiadi, and A. I. Rudskoy, “Nanostructure mechanism of formation of oxide film in heat-resistant Fe – 25Cr – 35Ni superalloys,” Met. Sci. Heat Treat., 56, 531 – 536 (2015).
M. M. Krishtal, P. V. Ivashin, A. V. Polunin et al., “Effect of SiO2 nanoparticles and soluble silicate on the composition and properties of oxide layers formed by microarc oxidizing on magnesium Mg96,” Met. Sci. Heat Treat., 61, 149 – 156 (2019).
Jiayun Zhang, Physical Chemistry of Metallurgy [in Chinese], Metallurgical Industry Press, Beijing, China (2004), p. 332 – 334.
Yutaka Ohya, Yusuke Ishii, and Takayuki Ban, “Reaction of molten aluminum with MgO and formation of MgAl2O4 spinel at 1000°C,” Mater. Trans., 61, 339 – 345 (2020).
Chao Jin, Jie Liu, Dongxing Zheng, et al., “Effect of cation substitution on the magnetic and magnetotransport properties of epitaxial Fe3–xVxO4 films,” Appl. Surf. Sci., 332, 70 – 75 (2015).
Weijun Huang, Min Chen, Xiang Shen, et al., “Phase diagrams and thermodynamic properties of the FeO – SiO2 – V2O3 systems,” Steel Res. Int., 88 (2017).
Qiuxia Cai, Jian-Guo Wang, Yong Wang, and Donghai Mei, “First-principles thermodynamics study of spinel MgAl2O4 surface stability,” J. Phys. Chem. C, 120, 19,087 – 19,096 (2106).
Nanqi Duan, Minrui Gao, Bin Hua, et al., “Exploring Ni(Mn1/3Cr2/3)2O4 spinel-based electrodes for solid oxide cells,” J. Mater. Chem. A, 8, 3988 – 3998 (2020).
Haiyan Li, Shengnan Sun, and Shibo Xi, “Metal-oxygen hybridization determined activity in spinel-based oxygen evolution catalysts: A case study of ZnFe2–xCrxO4,” Chem. Mater., 30, 6839 – 6848 (2018).
Yu Wang, Ai-Pin Jia, and Meng-Fei Luo, “Highly active spinel type CoCr2O4 catalysts for dichloromethane oxidation,” Appl. Catal. B-Environ., 165, 477 – 486 (2015).
The work has been supported financially by the National Natural Science Foundation of China (NSFC 51775289) and the Major Project of the Shandong Province Natural Science Foundation (ZR2018ZB0524).
Author information
Authors and Affiliations
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 10, pp. 23 – 29, October, 2021.
Rights and permissions
About this article
Cite this article
Wang, H., Sun, S. A Study of the Mechanism of Formation of Composite Oxide Scale on Heat-Resistant Steel 25Cr18Ni9Si2. Met Sci Heat Treat 63, 540–546 (2022). https://doi.org/10.1007/s11041-022-00725-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-022-00725-w