Abstract
Objective
The objective of this study was to assess the clinical response and safety of mirtazapine in the pediatric population with a diagnosis of functional nausea and nausea associated with functional dyspepsia postprandial distress syndrome.
Methods
This was a retrospective chart review to evaluate the safety and efficacy of mirtazapine for pediatric nausea and nausea associated with functional dyspepsia postprandial distress syndrome. Clinical response was classified as complete response, partial response, and no response. We also identified the prescribed doses, side effects, and weight changes during mirtazapine therapy.
Results
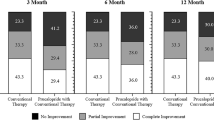
Among the 57 total patients, 67% were females and ages ranged from 7 to 19 years with a mean of 14 ± 3 years. Clinical (complete and partial) response was reported in 82% of patients. Nausea resolved in 82% and insomnia in 77% of the patients. Eighty-four percent gained weight with a mean of 4 ± 7 kg. Sixty-five percent did not report adverse effects. The most common adverse effects were undesired weight gain (16%) and dysphoria (9%). Two patients discontinued the medicine after the first dose because of adverse effects. There was a significant correlation between the initial dose and weight (rs = 0.478; p = 0.0002). The median initial and final doses were 15 mg, respectively.
Conclusions
Mirtazapine is an option for treating children and adolescents with functional nausea and nausea associated with functional dyspepsia post-prandial distress syndrome, especially for a select group of patients with concurrent weight loss, anxiety, and insomnia.


Similar content being viewed by others
References
Hyams J, Di Lorenzo C, Saps M, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2016;150:1456–68.
Jilani TN, Gibbons JR, Faizy RM, et al. Mirtazapine. [Updated 2021 Aug 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. https://www.ncbi.nlm.nih.gov/books/NBK519059/. Accessed 12 Dec 2021.
Tack J, Giao H, Carbone F, Vanheel H, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol. 2016;14:385-92.e4.
Carbone F, Vanuytsel T, Tack J. The effect of mirtazapine on gastric accommodation, gastric sensitivity distention, and nutrient tolerance in healthy subjects. Neurogastroenterol Motil. 2017;29(12):e13146.
Tack J, Carbone F. Functional dyspepsia and gastroparesis. Curr Opin Gastroenterol. 2017;33:446–54.
Malamood M, Roberts A, Kataria R, et al. Mirtazapine for symptom control in refractory gastroparesis. Drug Des Dev Ther. 2017;11:1035–41.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R-project.org/. Accessed 7 Dec 2021.
Pae C. Low-dose mirtazapine may be successful treatment option for severe nausea and vomiting. Progress in Neuro-Psychopharmacology & Biology Psychiatry. 2006;30:1143–5.
Thompson A, Lummis CRS. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007;11:527–40.
Jiang S, Jia L, Jing L, et al. Beneficial effects of antidepressant mirtazapine in functional dyspepsia patients. World J Gastroenterol. 2016;22:5260–6.
Coskun M, Alyanak B. Psychiatric co-morbidity and efficacy of mirtazapine treatment in young subjects with chronic or cyclic vomiting syndromes: a case series. J Neurogastroenterol Motil. 2011;17:305–11.
Karsten J, Hagenauw L, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomized, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327–37.
Mrakotsky C, Masek B, Biederman J, et al. Prospective open-label pilot trial of mirtazapine in children and adolescents with social phobia. J Anxiety Disord. 2008;22:88–97.
Hosoglu E, Herguner S. Childhood sleep terror and mirtazapine. J Child Adolesc Psychopharmacol. 2016;26:568.
Tanidir C, Herguner S. Mirtazapine for choking phobia: report of a pediatric case. J Child Adolesc Psychopharmacol. 2015;25:659–60.
Naviaux A. Management of Avoidant restrictive food intake disorder in a 12-year-old on a Pediatric ward in a General Hospital: Use of mirtazapine, partial hospitalization model and family-based therapy. Psychiatr Danub. 2019;31:421–6.
Doyle C, McDougle C. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues Clin Neurosci. 2012;14:263–79.
Hussain S, Hyman P. Psychotropic medications for pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2014;59:280–7.
Tack J, Janssen P, Masaoka T, et al. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol and Hepatol. 2012;10:1239–45.
Tack K, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol. 2018;43:111–7.
Bridge J, Iyengar S, Salary C, et al. Clinical response and risk for reported suicidal ideation and suicidal attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest/Competing interests
There is no financial disclosure or conflicts of interests.
Availability of data and material
Available upon request
Code availability
Not applicable
Ethics approval
LSU: #10116, date: 7-30-2018; CCHMC: #00002988, date: 7-30-2018
Consent to participate
Consent waivered for retrospective chart review
Consent for publication
Consent available
Authors' contributions
Dr. Iglesias-Escabi wrote the IRB proposal, reviewed charts, collected data, interpreted the collected data, and wrote the first draft of the manuscript with input from Dr. Santucci, Dr. Hyman, and Dr. LeBlanc. Dr. McDaniel performed statistical analysis for the study.Dr. Reuther provided psychology feedback, critically review the study proposal, drafted and revised the manuscript. Dr. Kleesattel reviewed charts, collected data, performed literature search and revised the manuscript. Dr. LeBlanc assisted in graphs and tables, drafted and revised the research work. (Late) Dr. Hyman conceptualized the study, provided feedback on study design, and contributed to revision of the manuscript. Dr. Santucci conceptualized the study, provided framework for study design, supervised and mentored Dr. Iglesias to prepare the manuscript and revised each iteration of the manuscript.
Rights and permissions
About this article
Cite this article
Iglesias-Escabi, I.M., Kleesattel, D., McDaniel, L.S. et al. Effect of Mirtazapine on Nausea in Children with Functional Nausea and Functional Dyspepsia Postprandial Distress Syndrome. Pediatr Drugs 24, 155–161 (2022). https://doi.org/10.1007/s40272-022-00494-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00494-2