Abstract
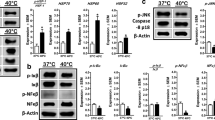
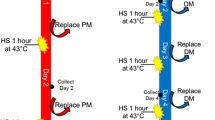
Short bouts of heat can induce a hormetic stress response, whereas prolonged or excessive exposure can elicit detrimental effects. We previously demonstrated an increase in autophagic signaling in C2C12 myotubes in response to 1 h of heat at 40 °C. In opposition, longer durations of heat exposure (e.g., 12 and 24 h) lead to an accumulation of autophagasomes and elevations in markers of cellular inflammation, oxidative stress, and apoptosis. Whether a longer, yet moderate, duration of 2 h of heat further enhances autophagic flux and attenuates stress and inflammatory signaling, or transitions the cell toward a dysregulation of autophagy is unclear. In this study, C2C12 myotubes were maintained at 37 °C or exposed to 40 °C (HT) for 2 h, and harvested immediately or following 2, 8, or 24 h of recovery. Two hours of HT immediately increased pAMPK (T172; p = 0.001), and subsequently increased pULK1 (S555) at 2 h of recovery (p = 0.028). LC3 II was increased at 8 h (p = 0.043) and 24 h (p = 0.015) of recovery, whereas p62 was elevated at 2 h (p = 0.002) and 8 h (p < 0.001) of recovery, but returned to baseline by 24 h. In Bafilomycin A1 treated cells, p62 was further increased immediately following HT (p = 0.041). There was also a significant elevation in p-p38 (Thr180/Try182), pJNK (Thr183/Tyr185), and pNFκB (Ser536). These findings suggest that as short as 2 h of heat exposure contributes to cell stress and accumulation of autophagasomes in skeletal muscle.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Mizushima, N., & Komatsu, M. (2011). Autophagy: renovation of cells and tissues. Cell, 147(4), 728–741.
Vainshtein, A., & Hood, D. A. (2016). The regulation of autophagy during exercise in skeletal muscle. Journal of Applied Physiology, 120(6), 664–673.
Masiero, E., et al. (2009). Autophagy is required to maintain muscle mass. Cell Metabolism, 10(6), 507–515.
Kim, Y. A., et al. (2013). Autophagic response to exercise training in skeletal muscle with age. Journal of Physiology and Biochemical, 69(4), 697–705.
Summers, C. M., & Valentine, R. J. (2019). Acute heat exposure alters autophagy signaling in C2C12 myotubes. Frontiers in Physiology, 10, 1521.
Liu, C. T., & Brooks, G. A. (2012). Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. Journal of Applied Physiology, 112(3), 354–361.
Gupte, A. A., et al. (2009). Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes, 58(3), 567–578.
Gupte, A. A., et al. (2011). Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. Journal of Applied Physiology, 110(2), 451–457.
Selsby, J. T., & Dodd, S. L. (2005). Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regulatory Integrative and Comparative Physiology, 289(1), R134–R139.
Selsby, J. T., et al. (2007). Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. Journal of Applied Physiology, 102(4), 1702–1707.
Naito, H., et al. (2000). Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. Journal of Applied Physiology, 88(1), 359–363.
Ganesan, S., et al. (2018). Short-term heat stress results in increased apoptotic signaling and autophagy in oxidative skeletal muscle in Sus scrofa. Journal of Thermal Biology, 72, 73–80.
Ganesan, S., et al. (2016). Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. American Journal of Physiology Regul Integr Comp Physiol, 310(11), R1288–R1296.
Montilla, S. I., et al. (2014). Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature, 1(1), 42–50.
Ganesan, S., et al. (2017). Short-term heat stress causes altered intracellular signaling in oxidative skeletal muscle. Journal of Animal Science, 95(6), 2438–2451.
Brownstein, A. J., et al. (2017) Heat stress causes dysfunctional autophagy in oxidative skeletal muscle. Physiological Reports, 5(12), e13317.
Russell, R. C., et al. (2013). ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nature Cell Biology, 15(7), 741–750.
Ganesan, S., et al. (2018). Short-term heat stress results in increased apoptotic signaling and autophagy in oxidative skeletal muscle in Sus scrofa. Journal of Thermal Biology, 72, 73–80.
Ganesan, S., et al. (2018). Prolonged environment-induced hyperthermia alters autophagy in oxidative skeletal muscle in Sus scrofa. Journal of Thermal Biology, 74, 160–169.
Neel, B. A., Lin, Y., & Pessin, J. E. (2013). Skeletal muscle autophagy: a new metabolic regulator. Trends in Endocrinology and Metabolism, 24(12), 635–643.
Jiang, J., et al. (2019). Targeting autophagy enhances heat stress-induced apoptosis via the ATP-AMPK-mTOR axis for hepatocellular carcinoma. International Journal of Hyperthermia, 36(1), 498–509.
McCormick, J. J., et al. (2020). Regulation of autophagy following ex vivo heating in peripheral blood mononuclear cells from young adults. Journal of Thermal Biology, 91, 102643.
Kumsta, C., et al. (2017). Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nature Communications, 8(1), 14337.
Klionsky, D. J., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition. Autophagy, 12(1), 1–222.
Rubinsztein, D. C., et al. (2009). In search of an “autophagomometer”. Autophagy, 5(5), 585–589.
Volodina, O., et al. (2017) Short-term heat stress alters redox balance in porcine skeletal muscle. Physiological Reports, 5(8), e13267.
Webber, J. L. (2010). Regulation of autophagy by p38alpha MAPK. Autophagy, 6(2), 292–293.
Slobodnyuk, K., et al. (2019). Autophagy-induced senescence is regulated by p38alpha signaling. Cell Death and Disease, 10(6), 376.
Schnoder, L., et al. (2016). Deficiency of neuronal p38alpha MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. Journal of Biology Chemistry, 291(5), 2067–2079.
Guo, F., et al. (2017). A central role for phosphorylated p38alpha in linking proteasome inhibition-induced apoptosis and autophagy. Molecular Neurobiology, 54(10), 7597–7609.
Sui, X., et al. (2014). p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Letters, 344(2), 174–179.
Ganesan, S., et al. (2017). Acute heat stress activated inflammatory signaling in porcine oxidative skeletal muscle. Physiological Reports, 5(16), e13397.
Zevian, S. C., & Yanowitz, J. L. (2014). Methodological considerations for heat shock of the nematode Caenorhabditis elegans. Methods, 68(3), 450–457.
Dokladny, K., Myers, O. B., & Moseley, P. L. (2015). Heat shock response and autophagy—cooperation and control. Autophagy, 11(2), 200–213.
McCormick, J. J., et al. (2021). Impaired autophagy following ex vivo heating at physiologically relevant temperatures in peripheral blood mononuclear cells from elderly adults. Journal of Thermal Biology, 95, 102790.
Brooks, G. A., et al. (1971). Tissue temperatures and whole-animal oxygen consumption after exercise. American Journal of Physiology, 221(2), 427–431.
Saltin, B., Gagge, A. P., & Stolwijk, J. A. (1968). Muscle temperature during submaximal exercise in man. Journal of Applied Physiology, 25(6), 679–688.
Metzger, K., et al. (2021). Effects of temperature on proliferation of myoblasts from donor piglets with different thermoregulatory maturities. BMC Molecular and Cell Biology, 22(1), 36.
Shima, A., & Matsuda, R. (2008). The expression of myogenin, but not of MyoD, is temperature-sensitive in mouse skeletal muscle cells. Zoological Science, 25(11), 1066–1074.
Powers, S. K., Howley, E. T., & Cox, R. (1982). A differential catecholamine response during prolonged exercise and passive heating. Medicine and Science in Sports and Exercise, 14(6), 435–439.
Welc, S. S., et al. (2013). Heat stroke activates a stress-induced cytokine response in skeletal muscle. Journal of Applied Physiology, 115(8), 1126–1137.
Welc, S. S., et al. (2012). Hyperthermia increases interleukin-6 in mouse skeletal muscle. American journal of physiology. Cell Physiology, 303(4), C455–C466.
King, M. A., et al. (2017). Unique cytokine and chemokine responses to exertional heat stroke in mice. Journal of Applied Physiology, 122(2), 296–306.
Hall, D. M., et al. (2001). Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol, 280(2), H509–H521.
Pearce, S. C., et al. (2014). Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. Journal of Animal Science, 92(12), 5444–5454.
Author information
Authors and Affiliations
Contributions
C.M.S.: Conceived and designed the study, performed experiments, analyzed data, drafted and edited the manuscript. R.J.V.: Conceived and designed the study, performed experiments, analyzed data, edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Summers, C.M., Valentine, R.J. Two hours of heat stress induces MAP-kinase signaling and autophagasome accumulation in C2C12 myotubes. Cell Biochem Biophys 80, 367–373 (2022). https://doi.org/10.1007/s12013-021-01054-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-01054-0