Abstract
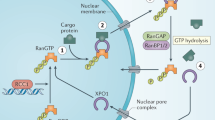
Nuclear export proteins such as exportin-1 (XPO1) transport tumor-suppressor proteins and other growth-regulatory proteins from the nucleus to the cytoplasm. Overexpression of XPO1 has been observed in several cancers and correlates with shorter event-free and overall survival in multiple myeloma. Selinexor was developed as an oral first-in-class selective inhibitor of nuclear export (SINE) that inhibits XPO1. Preclinical studies in tumor cell lines and mouse models have demonstrated the efficacy of selinexor both as a single agent and in various combinations with known active antimyeloma agents. Results from the pivotal phase II STORM trial led to the US FDA approval of selinexor with dexamethasone in penta-refractory myeloma. Because of the feasibility of combining selinexor with other active antimyeloma agents, the multiarm STOMP trial was initiated and is ongoing, with impressive response rates reported in some of the combination arms thus far. The registrational phase III BOSTON trial demonstrated the superiority of selinexor in combination with bortezomib and dexamethasone as compared with bortezomib and dexamethasone in patients with relapsed refractory multiple myeloma (RRMM) who have received one to three prior anti-MM regimens. The toxicity profile of selinexor is well established and predictable and may be significant unless managed aggressively and preemptively. The most common side effects are thrombocytopenia, anemia, neutropenia, fatigue, nausea, anorexia, and weight loss. Hyponatremia and cataracts seem to be class effects. Other SINE compounds are now being studied in efforts to discover agents that will potentially be better tolerated. Eltanexor is an investigational SINE compound that has shown a more positive toxicity profile in preclinical studies, with reduced central nervous system penetration and gastrointestinal side effects, and is now undergoing clinical investigation. These and other trials will further clarify the role of these innovative agents in the therapeutic advancement of RRMM.


Similar content being viewed by others
References
Xu D, Farmer A, Collett G, Grishin NV, Chook YM. Sequence and structural analyses of nuclear export signals in the NESdb database. Mol Biol Cell. 2012;23(18):3677–93. https://doi.org/10.1091/mbc.E12-01-0046.
Mahipal A, Malafa M. Importins and exportins as therapeutic targets in cancer. Pharmacol Ther. 2016;164:135–43. https://doi.org/10.1016/j.pharmthera.2016.03.020.
Sharpless NE, DePinho RA. Cancer biology: gone but not forgotten. Nature. 2007;445(7128):606–7. https://doi.org/10.1038/nature05567.
Culjkovic-Kraljacic B, Baguet A, Volpon L, Amri A, Borden KL. The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation. Cell Rep. 2012;2(2):207–15. https://doi.org/10.1016/j.celrep.2012.07.007.
Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KLB. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175(3):415–26. https://doi.org/10.1083/jcb.200607020.
Gravina GL, Senapedis W, McCauley D, Baloglu E, Shacham S, Festuccia C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7:85. https://doi.org/10.1186/s13045-014-0085-1.
Li S, Fu J, Lu C, Mapara MY, Raza S, Hengst U, Lentzsch S. Elevated translation initiation factor eIF4E is an attractive therapeutic target in multiple myeloma. Mol Cancer Ther. 2016;15(4):711–9. https://doi.org/10.1158/1535-7163.MCT-15-0798.
Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28(1):155–65. https://doi.org/10.1038/leu.2013.115.
Schmidt J, Braggio E, Kortuem KM, Egan JB, Zhu YX, Xin CS, et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia. 2013;27(12):2357–65. https://doi.org/10.1038/leu.2013.172.
Tan DS, Bedard PL, Kuruvilla J, Siu LL, Razak AR. Promising SINEs for embargoing nuclear-cytoplasmic export as an anticancer strategy. Cancer Discov. 2014;4(5):527–37. https://doi.org/10.1158/2159-8290.CD-13-1005.
Yoshimura M, Ishizawa J, Ruvolo V, Dilip A, Quintás-Cardama A, McDonnell TJ, et al. Induction of p53-mediated transcription and apoptosis by exportin-1 (XPO1) inhibition in mantle cell lymphoma. Cancer Sci. 2014;105(7):795–801. https://doi.org/10.1111/cas.12430.
Tiedemann RE, Zhu YX, Schmidt J, Shi CX, Sereduk C, Yin H, et al. Identification of molecular vulnerabilities in human multiple myeloma cells by RNA interference lethality screening of the druggable genome. Can Res. 2012;72(3):757–68. https://doi.org/10.1158/0008-5472.CAN-11-2781.
Chesi M, Matthews GM, Garbitt VM, Palmer SE, Shortt J, Lefebure M, et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120(2):376–85. https://doi.org/10.1182/blood-2012-02-412783.
Elloul S, Chang H, Klebanov B, Kashyap T, Werman M, Lee M, et al. Synergistic antitumor effect of selinexor, a selective inhibitor of nuclear export (SINE) compound and panobinostat in a mouse model of multiple myeloma. Cancer Res. 2016;76:4720.
Abdi J, Chen G, Chang H. Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget. 2013;4(12):2186–207. https://doi.org/10.18632/oncotarget.1497.
Turner JG, Dawson J, Cubitt CL, Baz R, Sullivan DM. Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol. 2014;27:62–73. https://doi.org/10.1016/j.semcancer.2014.03.001.
Rosebeck S, Kandarpa M, Alonge MM, Jasielec J, Dytfeld D, Maxwell SP, et al. Effects of inhibition of XPO1/CRM1-dependent nuclear export by selinexor (KPT-330), alone and in combination withcarfilzomib (CFZ), on apoptosis and autophagy in multiple myeloma (MM). Blood. 2013;122(21):279. https://doi.org/10.1182/blood.V122.21.279.279.
Turner JG, Kashyap T, Dawson JL, Gomez J, Bauer AA, Grant S, et al. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IκBα and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget. 2016;7(48):78896–909. https://doi.org/10.18632/oncotarget.12969.
Rosebeck S, Alonge MM, Kandarpa M, Mayampurath A, Volchenboum SL, Jasielec J, et al. Synergistic Myeloma Cell Death via Novel Intracellular Activation of Caspase-10-Dependent Apoptosis by Carfilzomib and Selinexor. Mol Cancer Ther. 2016;15(1):60–71. https://doi.org/10.1158/1535-7163.MCT-15-0488.
Turner JG, Dawson J, Bauer A, Gomez J, Baloglu E, Landesman Y, et al. Ixazomib combined with the nuclear export inhibitors selinexor or eltanexor for the treatment of multiple myeloma. Experimental and Molecular Therapeutics. AACR Annual Meeting 2019, Atlanta, GA. 2019; Abstract #285.
Lü S, Wang J. The resistance mechanisms of proteasome inhibitor bortezomib. Biomark Res. 2013;1(1):13. https://doi.org/10.1186/2050-7771-1-13.
Markovina S, Callander NS, O’Connor SL, Kim J, Werndli JE, Raschko M, et al. Bortezomib-resistant nuclear factor-kappaB activity in multiple myeloma cells. Mol Cancer Res. 2008;6(8):1356–64. https://doi.org/10.1158/1541-7786.MCR-08-0108.
Chanukuppa V, Paul D, Taunk K, Chatterjee T, Sharma S, Kumar S, et al. XPO1 is a critical player for bortezomib resistance in multiple myeloma: a quantitative proteomic approach. J Proteomics. 2019;209: 103504. https://doi.org/10.1016/j.jprot.2019.103504.
Nair J, Musi E, Schwartz GK. Selinexor (KPT-330) induces tumor suppression through nuclear sequestration of IκB and down-regulation of survivin. Clin. Cancer Res. 2017; 23(15): 4301–4311. https://doi.org/10.1158/1078-0432.CCR-16-2632.
Argueta C, Kashayp T, Klebanov B, Unger TJ, Guo C, Harrington S, et al. Selinexor synergizes with dexamethasone to repress mTORC1 signaling and induce multiple myeloma cell death. Oncotarget. 2018; 9(39): 25529–25544. https://doi.org/10.18632/oncotarget.25368.
Kashyap T, Argueta C, Unger T, Klebanov B, Debler S, Senapedis W, et al. Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget. 2018;9(56):30773–30786. https://doi.org/10.18632/oncotarget.25637.
Turner JG, Dawson JL, Grant S, Shain KH, Dalton WS, Dai Y, et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J. Hematol. Oncol. 2016; 9(1): 73. https://doi.org/10.1186/s13045-016-0304-z.
Turner JG, Cui Y, Bauer AA, Dawson JL, Gomez JA, Kim J, et al. Melphalan and Exportin 1 Inhibitors Exert Synergistic Antitumor Effects in Preclinical Models of Human Multiple Myeloma. Cancer Res. 2020; 80(23): 5344-5354. https://doi.org/10.1158/0008-5472.CAN-19-0677.
Chen C, Siegel D, Gutierrez M, Jacoby M, Hofmeister CC, Gabrail N, et al. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood. 2018; 131(8): 855–863. https://doi.org/10.1182/blood-2017-08-797886.
Vogl DT, Dingli D, Cornell RF, Huff CA, Jagannath S, Bhutani D, et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J. Clin. Oncol. 2018; 36(9): 859–866. https://doi.org/10.1200/JCO.2017.75.5207.
Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. Oral selinexor–dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 2019; 381: 727–738. https://doi.org/10.1056/NEJMoa1903455.
Gavriatopoulou M, Vogl DT, Nooka A, Dingli D, Cole C, Moreau P, et al. Effect of age on the safety and efficacy of selinexor in patients with relapsed refractory multiple myeloma: a post-hoc analysis of the STORM study. Clinical Lymphoma, Myeloma and Leukemia. 2019; 19(10): E117-E118. https://doi.org/10.1016/j.clml.2019.09.195
Vogl DT, Nooka A, Gavriatopoulou M, Yee A, Huff C, Moreau P, et al. Improvements in renal function with selinexor in relapsed/refractory multiple myeloma: post-hoc analyses from the STORM study. Clinical Lymphoma, Myeloma and Leukemia. 2019; 19(10): E118-E119. https://doi.org/10.1016/j.clml.2019.09.196.
Cornell R, Parameswaran H, Tang S, Barnstead A, Biran N, Callandar N, et al. Real world vs. clinical trial outcomes of triple class refractory penta-exposed multiple myeloma (MM). Clinical Lymphoma, Myeloma and Leukemia. 2019; 19(10): E115-E116. https://doi.org/10.1016/j.clml.2019.09.191.
Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38 targeted monoclonal antibody therapy. Leukemia. 2019; 33(9): 2266–2275. https://doi.org/10.1038/s41375-019-0435-7.
Richardson PG, Jagannath S, Chari A, Vogl DT, Dimopoulos MA, Moreau P et al. Overall Survival with oral selinexor plus low-dose dexamethasone versus real-world therapy in triple-class-refractory multiple myeloma. EJHaem. 2021; 2(1): 48–55. https://doi.org/10.1002/jha2.120.
Bahlis NJ, Sutherland H, White D, Sebag M, Lentzsch S, Kotb R, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018; 132(24): 2546–2554. https://doi.org/10.1182/blood-2018-06-858852.
Knopf KB, Duh MS, Lafeuille M-H, Gravel J, Lefebvre P, Nicolescu L et al. Meta-analysis of the efficacy and safety of bortezomib re-treatment in patients with multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2014; 14(5): 380–388. https://doi/10.16/j.clml.2014.03.005.
Jakubowiak AJ, Jasielec JK, Rosenbaum CA, Cole CE, Chari A, Mikhael J, et al. Phase I study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br. J. Haematol. 2019; 186(4): 549–560. https://doi.org/10.1111/bjh.15969.
Gasparetto C, Schiller G, Tuchman S, Callander N, Baljevic M, Lentzsch S. et al. Once weekly selinexor, carfilzomib, and dexamethasone (xkd) in carfilzomib nonrefractory multiple myeloma (mm) patients. EHA June 2021 abstract # S188.
White D, LeBlanc R, Venner C, Bahlis NJ, Lentzsch S, Gasparetto C, et al. Safety and efficacy of the combination of selinexor, lenalidomide and dexamethasone (SRd) in patients with relapsed/refractory multiple myeloma (RRMM). 17th Int. Myeloma Work. 84–86, OAB-083 (2019).
White DJ, LeBlanc R, Baljevic M, Bahlis NJ, Lentzsch S, Venner CP, et al. Selinexor, Lenalidomide and Dexamethasone (SRd) for Patients with Relapsed/Refractory and Newly Diagnosed Multiple Myeloma. Blood 2020. 136 (Supplement 1): 45–46. https://doi.org/10.1182/blood-2020-140141.
White D, Chen C, Baljevic, M, Tuchman S, Bahlis NJ, Schiller GJ, et al. Oral selinexor, pomalidomide, and dexamethasone (XPd) at recommended phase 2 dose in relapsed refractory multiple myeloma (MM). Journal of Clin Oncol. 2021; 39 (15): 8018.https://doi.org/10.1200/JCO.2021.39.15_suppl.8018.
Gasparetto CJ, Lentzsch S, Schiller GJ, Callander N, Tuchman S, Chen C, et al. Selinexor, daratumumab, and dexamethasone (SDd) in patients with relapsed or refractory multiple myeloma. European Journal of Haematology 2021; 2: 56-65. https://doi.org/10.1002/jha2.122.
Baz R, Zonder JA, Shain KH, Alsina M, Brayer JB, Melody M, et al. Phase I/II Study of Liposomal Doxorubicin (DOX) in Combination with Selinexor (SEL) and Dexamethasone (Dex) for Relapsed and Refractory Multiple Myeloma (RRMM). Blood 2017; 130 (Supplement 1): 3095. https://doi.org/10.1182/blood.V130.Suppl_1.3095.3095.
Nishihori T, Alsina M, Ochoa J, Puglianini OAC, Baz R, Shain KH, et al. The Result of a Phase 1 Study of Selinexor in Combination with High-Dose Melphalan and Autologous Hematopoietic Cell Transplantation for Multiple Myeloma. Blood. 2019; 134 (Supplement_1): 3314. https://doi.org/10.1182/blood-2019-131321.
Grosicki S, Simonova M, Spicka I, Pour L, Kriachok I, Gavriatopoulou M, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. The Lancet. 2020; 396(10262): P1563-1573. https://doi.org/10.1016/S0140-6736(20)32292-3.
Richard S, Chari A, Delimpasi S, Simonova M, Spicka I, Pour L, et al. Selinexor, bortezomib, and dexamethasone versus bortezomib and dexamethasone in previously treated multiple myeloma: Outcomes by cytogenetic risk. American Journal of Hematol. 2021; 96: 1120-1130. https://doi.org/10.1002/ajh.26261.
Lassman A, Wen P, Bent V, Plotkin S, Walenkamp A, Huang X, et al. Efficacy and safety of selinexor in recurrent glioblastoma. J Clin Oncol. 2019; 37(Suppl 15): 2005. https://doi.org/10.1200/JCO.2019.37.15.
Machlus KR, Wu SK, Vijey P, Soussou TS, Liu Z-J, Shacham E, et al. Selinexor-induced thrombocytopenia results from inhibition of thrombopoietin signaling in early megakaryopoiesis. Blood. 2017; 130(9): 1132–1143.https://doi.org/10.1182/blood-2016-11-752840.
Hing ZA, Fung HYJ, Ranganathan P, Mitchell S, El-Gamal D, Woyach JA, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 2016;30(12):2364–72. https://doi.org/10.1038/leu.2016.136.
Turner J, Dawson J, Cubitt C, Baluglo E, Grant S, Dai Y, et al. Next generation XPO1 inhibitor KPT-8602 for the treatment of drug-resistant multiple myeloma. Blood. 2015; 126(23): 1818. https:// doi.org/https://doi.org/10.1182/blood.V126.23.1818.1818.
Cornell RF, Rossi A, Baz R, Hofmeister CC, Shustik C, Richter JR, et al. Eltanexor (KPT-8602), a Second Generation Selective Inhibitor of Nuclear Export (SINE) Compound, in Patients with Refractory Multiple Myeloma. Blood. 2017; 130 (1): 3134. https://doi.org/10.1182/blood.V130.Suppl_1.3134.3134.
Hamamoto T, Seto H, Beppu T. Leptomycins A and B, new antifungal antibiotics II. Structure elucidation. J Antibiot (Tokyo). 1983; 36(6): 646–50. https://doi.org/10.7164/antibiotics.36.646.
Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994; 269(9): 6320–4. https://doi.org/10.1016/S0021-9258(17)37374-X.
Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner E, Wolff B, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999; 96(16): 9112–7. https://doi.org/10.1073/pnas.96.16.9112.
Roberts BJ, Hamelehle KL, Sebolt JS, Leopold WR. In vivo and in vitro anticancer activity of the structurally novel and highly potent antibiotic CI-940 and its hydroxy analog (PD 114,721). Cancer Chemother Pharmacol. 1986; 16(2): 95–101.https://doi.org/10.1007/BF00256156.
Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. Br J Cancer. 1996; 74(4): 648–9. https://doi.org/10.1038/bjc.1996.415.
Sakakibara K, Saito N, Sato T, Suzuki A, Hasegawa Y, Friedman J, et al. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood. 2011; 118(14): 3922–31. https://doi.org/10.1182/blood-2011-01-333138.
Chen J, Brooks CL, McDonald P, Schwartz JD, Schneider RS, Sakakibara K, et al. SL-801, a Novel, Reversible Inhibitor of Exportin-1 (XPO1) / Chromosome Region Maintenance-1 (CRM1) with Broad and Potent Anti-Cancer Activity. Blood (2015) 126 (23): 4433. https://doi.org/10.1182/blood.V126.23.4433.4433.
Wang J, Barve M, Chiorean E, LoRusso P, Courtney K, Qi D, et al. Interim results from trial of SL-801, a novel XPO-1 inhibitor, in patients with advanced solid tumours. Ann Oncol. 2019; 30(Suppl 5): v175. https://doi.org/10.1093/annonc/mdz244.028.
Wang S, Sellner L, Wang L, Sauer T, Neuber B, Gong W, et al. Combining selective inhibitors of nuclear export (SINEs) with chimeric antigen receptor (CAR) T cells for CD19-positive malignancies. Oncology Reports. 2021; 46 (2): 170. https://doi.org/10.3892/or.2021.8121.
Chari A, Vogl DT, Jagannath S, Jasielec JK, DeCastro A, Unger TJ, et al. Selinexor-containing regimens for the treatment of patients with multiple myeloma refractory to chimeric antigen receptor T-cell (CAR-T) Therapy. Blood. 2019; 134 (Suppl 1): 1854. https://doi.org/10.1182/blood-2019-128887.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Dr. Richard has served as a consultant for Karyopharm Therapeutics and Bristol-Myers Squibb and has received honoraria from Janssen Pharmaceuticals. Dr. Jagannath is a consultant for Bristol-Myers Squibb, Janssen Pharmaceuticals, Karyopharm Therapeutics, Legend Biotech, Takeda, and Sanofi.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Both authors have contributed equally to the work on the manuscript.
Rights and permissions
About this article
Cite this article
Richard, S., Jagannath, S. Targeting Nuclear Export Proteins in Multiple Myeloma Therapy. BioDrugs 36, 13–25 (2022). https://doi.org/10.1007/s40259-021-00514-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-021-00514-6