Abstract
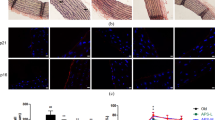
Vascular endothelial cells play a vital role in atherosclerotic changes and the progression of cardiovascular disease in older adults. Previous studies have indicated that Astragalus polysaccharides (APS), a main active component of the traditional Chinese medicine Astragalus, protect mitochondria and exert an antiaging effect in the mouse liver and brain. However, the effect of APS on rat aortic endothelial cell (RAEC) senescence and its underlying mechanism have not been investigated. In this study, we extracted RAECs from 2-month-old male Wistar rats by the tissue explant method and found that APS ameliorated the high-glucose-induced increase in the frequency of SA-β-Gal positivity and the levels of the senescence-related proteins p16, p21, and p53. APS increased the tube formation capacity of RAECs under high-glucose conditions. Moreover, APS enhanced the expression of the mitochondrial Na+/Ca2+ exchanger NCLX, and knockdown of NCLX by small interfering RNA (siRNA) transfection suppressed the antiaging effect of APS under high-glucose conditions. Additionally, APS ameliorated RAEC mitochondrial dysfunction, including increasing ATP production, cytochrome C oxidase activity and the oxygen consumption rate (OCR), and inhibited high-glucose-induced NLRP3 inflammasome activation and IL-1β release, which were reversed by siNCLX. These results indicate that APS reduces high-glucose-induced inflammasome activation and ameliorates mitochondrial dysfunction and senescence in RAECs by modulating NCLX. Additionally, APS enhanced the levels of autophagy-related proteins (LC3B-II/I, Atg7) and increased the quantity of autophagic vacuoles under high-glucose conditions. Therefore, these data demonstrate that APS may reduce vascular endothelial cell inflammation and senescence through NCLX.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- APS:
-
Astragalus polysaccharides
- RAECs:
-
Rat aortic endothelial cells
- SA-β-gal:
-
Senescence-associated β-galactosidase
- IL-1β:
-
Interleukin-1β
- CVD:
-
Cardiovascular diseases
- ROS:
-
Reactive oxygen species
- NLRP3:
-
NOD-like receptor family pyrin domain-containing 3
- ECL:
-
Enhanced chemiluminescence
- IgG:
-
Immunoglobulin G
- Con:
-
Control
- HG:
-
High glucose
- ATP:
-
Adenosine triphosphate
- COX:
-
Cytochrome c oxidase
- OCR:
-
Oxygen consumption rate
- FCCP:
-
carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- Atg:
-
Autophagy
References
Theodorou, K., & Boon, R. A. (2018). Endothelial cell metabolism in atherosclerosis. Frontiers in Cell and Developmental Biology, 6, 82.
Das, A., Huang, G. X., Bonkowski, M. S., Longchamp, A., Li, C., Schultz, M. B., Kim, L. J., Osborne, B., Joshi, S., Lu, Y., Treviño-Villarreal, J. H., Kang, M. J., Hung, T. T., Lee, B., Williams, E. O., Igarashi, M., Mitchell, J. R., Wu, L. E., Turner, N., Arany, Z., Guarente, L., & Sinclair, D. A. (2019). Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell, 176, 944–945.
E, S., Kijima, R., Honma, T., Yamamoto, K., Hatakeyama, Y., Kitano, Y., Kimura, T., Nakagawa, K., Miyazawa, T., & Tsuduki, T. (2014). 1-Deoxynojirimycin attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells. Experimental Gerontology, 55, 63–69.
Zhao, Q., Gao, C., & Cui, Z. (2015). Ginkgolide A reduces inflammatory response in high-glucose-stimulated human umbilical vein endothelial cells through STAT3-mediated pathway. International Immunopharmacology, 25, 242–248.
Carafoli, E., Tiozzo, R., Lugli, G., Crovetti, F., & Kratzing, C. (1974). The release of calcium from heart mitochondria by sodium. Journal of Molecular and Cellular Cardiology, 6, 361–371.
Palty, R., Silverman, W. F., Hershfinkel, M., Caporale, T., Sensi, S. L., Parnis, J., Nolte, C., Fishman, D., Shoshan-Barmatz, V., Herrmann, S., Khananshvili, D., & Sekler, I. (2010). NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proceedings of the National Academy of Sciences of the United States of America, 107, 436–441.
Zu, Y., Wan, L. J., Cui, S. Y., Gong, Y. P., & Li, C. L. (2015). The mitochondrial Na+/Ca2+ exchanger may reduce high glucose-induced oxidative stress and nucleotide-binding oligomerization domain receptor 3 inflammasome activation in endothelial cells. Journal of Geriatric Cardiology, 12, 270–278.
Liu, P., Zhao, H., & Luo, Y. (2017). Anti-aging implications of Astragalus Membranaceus (Huangqi): a well-known Chinese tonic. Aging and Disease, 8, 868–886.
Huang, W. M., Liang, Y. Q., Tang, L. J., Ding, Y. & Wang, X. H. (2013). Antioxidant and anti-inflammatory effects of Astragalus polysaccharide on EA.hy926 cells. Experimental and Therapeutic Medicine., 6, 199–203.
Yang, L. P., Shen, J. G., Xu, W. C., Li, J., & Jiang, J. Q. (2013). Secondary metabolites of the genus Astragalus: structure and biological-activity update. Chemistry & Biodiversity,, 10, 1004–1054.
Zhou, Y., Hong, T., Tong, L., Liu, W., Yang, X., Luo, J., & Yan, L. (2018). Astragalus polysaccharide combined with 10-hydroxycamptothecin inhibits metastasis in non-small cell lung carcinoma cell lines via the MAP4K3/mTOR signaling pathway. International Journal of Molecular Medicines, 42(6), 3093–3104.
Sun, S., Yang, S., Dai, M., Jia, X., Wang, Q., Zhang, Z., & Mao, Y. (2017). The effect of Astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose -stimulated H9C2 cells. BMC Complementary Alternative Medicines, 17(1), 310.
Wang, P., Zhang, Z., Ma, X., Huang, Y., Liu, X., Tu, P., & Tong, T. (2003). HDTIC-1 and HDTIC-2, two compounds extracted from Astragali Radix, delay replicative senescence of human diploid fibroblasts. Mechanisms of Ageing and Development, 124, 1025–1034.
Li, X. T., Zhang, Y. K., Kuang, H. X., Jin, F. X., Liu, D. W., Gao, M. B., Liu, Z., & Xin, X. J. (2012). Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. International Journal of Molecular Sciences, 13, 1747–1761.
Yang, F., Yan, G., Li, Y., Han, Z., Zhang, L., Chen, S., Feng, C., Huang, Q., Ding, F., Yu, Y., Bi, C., Cai, B., & Yang, L. (2016). Astragalus polysaccharide attenuated iron overload-induced dysfunction of mesenchymal stem cells via suppressing mitochondrial ROS. Cellular Physiology and Biochemistry, 39, 1369–1379.
Zhu, X. X., Miao, X. Y., Gong, Y. P., Fu, B., & Li, C. L. (2019). Isolation and culture of rat aortic endothelial cells in vitro: a novel approach without collagenase digestion. Journal of Cellular Biochemistry, 120, 14127–14135.
Yau, W. W., Singh, B. K., Lesmana, R., Zhou, J., Sinha, R. A., Wong, K. A., Wu, Y., & Yen, P. M. (2019). Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy, 15, 131–150.
Miao, X., Gu, Z., Liu, Y., Jin, M., Lu, Y., Gong, Y., Li, L., & Li, C. (2018). The glucagon-like peptide-1 analogue liraglutide promotes autophagy through the modulation of 5’-AMP-activated protein kinase in INS-1 β-cells under high glucose conditions. Peptides, 100, 127–139.
Weinberg, R. A. (1995). The retinoblastoma protein and cell cycle control. Cell, 81, 323–330.
Morgan, R. G., Ives, S. J., Lesniewski, L. A., Cawthon, R. M., Andtbacka, R. H., Noyes, R. D., Richardson, R. S., & Donato, A. J. (2013). Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. American Journal of Physiology-Heart and Circulatory Physiology, 305, H251–258.
Goldberg, E. L., & Dixit, V. D. (2015). Drivers of age-related inflammation and strategies for healthspan extension. Immunological Reviews, 265, 63–74.
Yin, Y., Zhou, Z., Liu, W., Chang, Q., Sun, G., & Dai, Y. (2017). Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. The International Journal of Biochemistry & Cell Biology, 84, 22–34.
Vion, A. C., Kheloufi, M., Hammoutene, A., Poisson, J., Lasselin, J., Devue, C., Pic, I., Dupont, N., Busse, J., Stark, K., Lafaurie-Janvore, J., Barakat, A. I., Loyer, X., Souyri, M., Viollet, B., Julia, P., Tedgui, A., Codogno, P., Boulanger, C. M., & Rautou, P. E. (2017). Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proceedings of the National Academy of Sciences of the United States of America, 114, E8675–E8684.
Choi, A. M., Ryter, S. W., & Levine, B. (2013). Autophagy in human health and disease. The New England Journal of Medicine, 368, 651–662.
Molyneux, R. J., & James, L. F. (1982). Loco intoxication: indolizidine alkaloids of spotted locoweed (Astragalus lentiginosus). Science, 216, 190–191.
Lai, M., Wang, J., Tan, J., Luo, J., Zhang, L. M., Deng, D. Y., & Yang, L. (2017). Preparation, complexation mechanism and properties of nano-complexes of Astragalus polysaccharide and amphiphilic chitosan derivatives. Carbohydrate Polymers, 161, 261–269.
Song, J., Chen, M., Li, Z., Zhang, J., Hu, H., Tong, X., & Dai, F. (2019). Astragalus polysaccharide extends lifespan via mitigating endoplasmic reticulum stress in the Silkworm, Bombyx mori. Aging & Disease, 10(6), 1187–1198.
Wang, N., Liu, J., Xie, F., Gao, X., Ye, J. H., Sun, L. Y., Wei, R., & Ai, J. (2015). miR-124/ATF-6, a novel lifespan extension pathway of Astragalus polysaccharide in Caenorhabditis elegans. Journal of Cellular Biochemistry, 116, 242–251.
Zhang, H., Pan, N., Xiong, S., Zou, S., Li, H., Xiao, L., Cao, Z., Tunnacliffe, A., & Huang, Z. (2012). Inhibition of polyglutamine-mediated proteotoxicity by Astragalus membranaceus polysaccharide through the DAF-16/FOXO transcription factor in Caenorhabditis elegans. Biochemical Journal,, 441, 417–424.
Childs, B. G., Bussian, T. J., & Baker, D. J. (2019). Cellular identification and quantification of senescence-associated β-galactosidase activity in vivo. Methods in Molecular Biology, 1896, 31–38.
Campisi, J. (2013). Aging, cellular senescence, and cancer. The Annual Review of Physiology, 75, 685–705.
Khananshvili, D. (2013). The SLC8 gene family of sodium-calcium exchangers (NCX) - structure, function, and regulation in health and disease. Molecular Aspects of Medicine, 34, 220–235.
Takeuchi, A., Kim, B., & Matsuoka, S. (2013). The mitochondrial Na+-Ca2+ exchanger, NCLX, regulates automaticity of HL-1 cardiomyocytes. Scientific Reports, 3, 2766.
Chistiakov, D. A., Sobenin, I. A., Revin, V. V., Orekhov, A. N., & Bobryshev, Y. V. (2014). Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Research International, 2014, 238463.
Broniarek, I., Dominiak, K., Galganski, L., & Jarmuszkiewicz, W. (2020). The influence of statins on the aerobic metabolism of endothelial cells. International Journal of Molecular Sciences, 21, 1485.
Zhu, W., Yuan, Y., Liao, G., Li, L., Liu, J., Chen, Y., Zhang, J., Cheng, J., & Lu, Y. (2018). Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death & Disease, 9, 837.
Fukuda, R., Zhang, H., Kim, J. W., Shimoda, L., Dang, C. V., & Semenza, G. L. (2007). HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell, 129, 111–122.
Kim, D., Sankaramoorthy, A., & Roy, S. (2020). Downregulation of Drp1 and Fis1 Inhibits mitochondrial fission and prevents high glucose-Induced apoptosis in retinal endothelial cells. Cells, 9, 1662.
Donato, A. J., Morgan, R. G., Walker, A. E., & Lesniewski, L. A. (2015). Cellular and molecular biology of aging endothelial cells. Journal of Molecular and Cellular Cardiology, 89, 122–135.
Donato, A. J., Machin, D. R., & Lesniewski, L. A. (2018). Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circulation Research, 123, 825–848.
Liu, Y., Shi, S., Gu, Z., Du, Y., Liu, M., Yan, S., Gao, J., Li, J., Shao, Y., Zhong, W., Chen, X., & Li, C. (2013). Impaired autophagic function in rat islets with aging. Age, 35, 1531–1544.
Meng, Q., Du, X., Wang, H., Gu, H., Zhan, J., & Zhou, Z. (2017). Astragalus polysaccharides inhibits cell growth and pro-inflammatory response in IL-1β-stimulated fibroblast-like synoviocytes by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Apoptosis, 22, 1138–1146.
Cao, Y., Shen, T., Huang, X., Lin, Y., Chen, B., Pang, J., Li, G., Wang, Q., Zohrabian, S., Duan, C., Ruan, Y., Man, Y., Wang, S., & Li, J. (2017). Astragalus polysaccharide restores autophagic flux and improves cardiomyocyte function in doxorubicin-induced cardiotoxicity. Oncotarget, 8, 4837–4848.
Acknowledgements
The authors would like to express their sincere gratitude to all the researchers at the Chinese PLA Institute of Nephrology, State Key Laboratory of Kidney Diseases. This study was supported by grants from the National Natural Science Foundation of China (No. 81774119, 81300265, 81900765); Special Scientific Research Project of Military Healthcare (19BJZ29), and Beijing Haidian District Health Development Research and Cultivation Program (No. HP2021-03-80303).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Miao, XY., Zhu, XX., Gu, ZY. et al. Astragalus Polysaccharides Reduce High-glucose-induced Rat Aortic Endothelial Cell Senescence and Inflammasome Activation by Modulating the Mitochondrial Na+/Ca2+ Exchanger. Cell Biochem Biophys 80, 341–353 (2022). https://doi.org/10.1007/s12013-021-01058-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-01058-w