Abstract
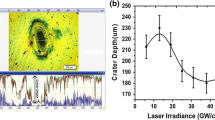
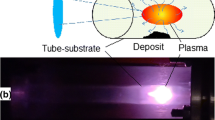
Laser induced dielectric breakdown (LIDB) on a surface of solid Mo in H2/BF3 atmosphere at 30–760 Torr and in a gaseous mixture MoF6/H2/BF3 + at 760 Torr pressure is tested for synthesis and deposition of superhard molybdenum borides that are needed in many areas of industry and technology. The emission spectra of the plasma and the dynamics of the gas discharge near the substrate are investigated. A comparative analysis of the gas mixture before and after exposure to LIDB plasma is carried out using IR spectroscopy. The conditions for the formation of molybdenum borides are determined. A thermodynamic analysis of the MoF6/H2/BF3 and Mo/H2/BF3 systems is carried out to determine the temperature range for the formation of molybdenum borides and establish the main chemical reactions responsible for their formation. Deposits containing MoB and MoB2 phases are obtained. For the mixture MoF6/H2/BF3, the deposit exhibits an amorphous layered structure, which contains 19.15 wt% F, 30.45% O, and 0.8% Si. For the Mo/H2/BF3 system at the pressures 30 and 160 Torr, nanopowder of molybdenum boride is produced with a characteristic grain size of 100 nm. At pressures above 160 Torr, Mo nanopowder with a grain size < 30 nm is obtained.








Similar content being viewed by others
References
Kaner RB, Gilman JJ, Tolbert SH (2005) Science 308:1268–1269
Chung HY, Weinberger MB, Levine JB et al (2007) Science 316:436–439
Steiniz R, Binder I, Moskowite D (1952) J Met 4:983–987
Levine JB, Tolbert SH, Kaner RB (2009) Adv Functional Mater 19:3519–3533
Zhang M, Wang H, Wang H, Cui T, Ma Y (2010) J Phys Chem C 114:6722–6725
Camurlu HE (2011) J Alloys Compounds 509:5431–5436
Barnett S, Madan A (1998) Phys World 11:45–48
Li Y, Fan Y, Chen Y (2003) J Solid State Chem 170:135–141
Jothi PR, Yubuta K, Fokwa BPT (2018) Adv Mater 30:1704181
Rybkovskiy DV, Kvashnin AG, Kvashnina YuA, Oganov AR (2020) Chem Mater 32:7028–7035
Kornev RA, Sennikov PG, Konychev DA et al (2016) J Radioanal Nucl Chem 309:833–840
Kornev RA, Sennikov PG, Nazarov VV (2017) Plasma Physics and Technology 4:169–172
Sennikov PG, Gornushkin IB, Kornev RA et al (2021) Plasma Chem Plasma Processing 41:673–690
Sennikov PG, Golubev SV, Mochalov LA, Kornev RA, Beliantsev SI, Zyryanov SM, Kossyu IA, Davydov AM (2017) Patent RF, 2610 583 [in Russian]
Sennikov PG, Kornev RA, Shishkin AI (2017) Plasma Chem Plasma Processing 37:997–1008
Kornev RA, Sennikov PG, Shabarova LV, Shishkin AI, Drozdova TA, Sintsov SV (2019) High Energy Chem 53:246–253
Kornev RA, Konychev DA, Sennikov PG, Zyryanov SM (2018) Patent RF № 2 648 421 [in Russian]
Jervis TR (1985) J Appl Phys 58:1400–1401
Jervis TR, Newkirk LR (1986) J Mater Res 1:420–424
Ronn AM (1976) Chem Phys Lett 42:202–204
Lin ST, Ronn AM (1978) Chem Phys Lett 56:414–418
Draper CW (1980) Met Trans 11A:349–351
Shin SM, Draper CW, Mochel ME, Rigsbee JM (1985) Materials Lett 3:265–269
Draper CW (1980) J Phys Chem 84:2089–2090
Gornushkin IB, Sennikov PG, Kornev RA, Ermakov AA, Shkrunin VE (2020) Plasma Chem Plasma Processing 40:1145–1162
Gribov LA, Smirnov VN (1961) Usp Fiz Nauk 527:527–567 [in Russian]
Shabarova LV, Plekhovich AD, Kutyin AM, Sennikov PG, Kornev RA (2019) High Energy Chem 53:155–161
Belov SG, Iorish VS, Yungman VS (2000) High Trmperature 38:191–196
Stern KH, Weise EL (1966) High Temperature Properties and Decomposition of Inorganic Salts, NSRDS- NBS7 (Washington D.C.: US Gov Print Office)
Gurvich LV, Veitz IV, Alcock CB (1989) Thermodynamic Properties of Individual Substances (New York: Hemisphere 21)
Gordon S, McBride BJ (1994) Computer program for calculation of complex chemical equilibrium compositions and application (NASA Reference Publication 1311) Online version: https://cearun.grc.nasa.gov
Parthiban S, Martin JML (2001) J Chem Phys 114:6014–6029
Çamurlu HE (2011) J Alloy Compd 509:5431–5436
Nuclear Sci. (1964) Abstracts 18 Oak Ridge Directed Operations, Technical Information Division 3785
Yeh CL, Hsu WS (2008) J Alloy Compd 457:191–197
Linn ST, Ronn AM (1978) Chem Phys Lett 56:414–418
Ronn AM, Earl BL (1977) Chem Phys Lett 45:556–558
Krikorian OH (1971) Lawrence Livermore National Laboratory Report UCRL- 51043
Blinder AV, Bolgar AS (1991) Powder Metall Met Ceram 30:1053–1056
Pankratz LB (1994) Thermodynamic Properties of Carbides, Nitrides, and Other Selected Substances Washington D.C. report 523
Meschel SV, Kleppa OJ (1993) Metall Mater Trans A 24:947–950
(1964) Tables of TD data, Vienna
Touloukian YS, Ho CY (1970) Thermophysical Properties of Matter TPRC Data Series 5
Lavut EG, Chelovskaya NV, Kashireninov OE (1993) J Eng Phys Thermophys 65:971–973
Morishita M, Koyama K, Yagi S (2004) J Alloy Compd 376:111–114
Gauthier MM (1995) Engineered Materials Handbook Desk Edition ASM Intl. 953
Acknowledgements
The authors are very grateful to the RSF Grant No 20-13-00035 basic support. as well as the Russian Ministry of Education and Science (subject 0095-2019-0008) for the partial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
A.Kornev, R., Sennikov, P.G., Gornushkin, I.B. et al. Laser Induced Dielectric Breakdown as a Novel Method for the Synthesis of Molybdenum Boride. Plasma Chem Plasma Process 42, 395–412 (2022). https://doi.org/10.1007/s11090-021-10224-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10224-0