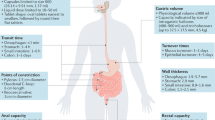
Abstract
Most pharmaceuticals are given using short-acting formulations that require frequent administration, which can negatively affect patient compliance and increase failure risks associated with inconsistent use. By contrast, long-acting release formulations can achieve sustained release of drugs for weeks, months or years. In this Review, we discuss long-acting drug delivery formulations that release drugs for at least 1 month and that have received approval from the US Food and Drug Administration (FDA), with an emphasis on materials used in their formulation. We highlight different slow-release mechanisms, including dissolution-based, biodegradation-based (preformed and in situ-formed), non-degradable implantable and hydrogel-based formulations, and investigate the clinical applications of long-acting drug delivery formulations, including long-acting contraceptives, extended sex hormone suppression, opioid and alcohol addiction treatments and localized drug delivery to the eye. Finally, we summarize release mechanisms, delivery duration, pharmaceutical forms, administration routes, indications, manufacturers and inactive ingredients of 63 FDA-approved long-acting drug products. We conclude by looking at the future challenges and opportunities for long-acting drug delivery formulations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Allen, L. V. & Ansel, H. C. in Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems 10th edn (Lippincott Williams & Wilkins, 2017).
Osterberg, L. & Blaschke, T. Adherence to medication. N. Engl. J. Med. 353, 487–497 (2005).
Zhao, Z., Ukidve, A., Kim, J. & Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 181, 151–167 (2020).
Yun, Y. H., Lee, B. K. & Park, K. Controlled drug delivery: historical perspective for the next generation. J. Control. Release 219, 2–7 (2015).
Park, K. et al. Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. J. Control. Release 304, 125–134 (2019).
Burgess, D. J. & Wright, J. C. in Long Acting Injections and Implants (eds Wright, J. C. & Burgess, D. J.) 1–9 (Springer, 2012).
Sun, Y., Scruggs, D. W., Peng, Y., Johnson, J. R. & Shukla, A. J. Issues and challenges in developing long-acting veterinary antibiotic formulations. Adv. Drug Deliv. Rev. 56, 1481–1496 (2004).
Wright, J. C. & Hoffman, A. S. in Long Acting Injections and Implants (eds Wright, J. C. & Burgess, D. J.) 11–24 (Springer, 2012).
Morishita, M. & Peppas, N. A. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today 11, 905–910 (2006).
Herwadkar, A. & Banga, A. K. Peptide and protein transdermal drug delivery. Drug Discov. Today Technol. 9, e147–e154 (2012).
Adjei, A. & Gupta, P. Pulmonary delivery of therapeutic peptides and proteins. J. Control. Release 29, 361–373 (1994).
Keizer, R. J., Huitema, A. D., Schellens, J. H. & Beijnen, J. H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 49, 493–507 (2010).
Shi, L., Hodges, M., Yurgin, N. & Boye, K. S. Impact of dose frequency on compliance and health outcomes: a literature review (1966–2006). Expert Rev. Pharmacoecon. Outcomes Res. 7, 187–202 (2007).
US Food and Drug Administration. Lupron Depot. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=019732 (2014).
Siegel, S. J. Extended release drug delivery strategies in psychiatry: theory to practice. Psychiatry 2, 22–31 (2005).
Park, E. J. et al. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch. Pharm. Res. 36, 651–659 (2013).
Haddad, P. M., Brain, C. & Scott, J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat. Outcome Meas. 5, 43–62 (2014).
Bangura, J. B., Xiao, S., Qiu, D., Ouyang, F. & Chen, L. Barriers to childhood immunization in sub-Saharan Africa: a systematic review. BMC Public Health 20, 1108 (2020).
Flexner, C. HIV drug development: the next 25 years. Nat. Rev. Drug Discov. 6, 959–966 (2007).
Blumenthal, P. D., Voedisch, A. & Gemzell-Danielsson, K. Strategies to prevent unintended pregnancy: increasing use of long-acting reversible contraception. Hum. Reprod. Update 17, 121–137 (2011).
Weiser, J. R. & Saltzman, W. M. Controlled release for local delivery of drugs: barriers and models. J. Control. Release 190, 664–673 (2014).
Jacob, S., Nair, A. B. & Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 24, 3 (2020).
Ferguson, G. T., Hickey, A. J. & Dwivedi, S. Co-suspension delivery technology in pressurized metered-dose inhalers for multi-drug dosing in the treatment of respiratory diseases. Respir. Med. 134, 16–23 (2018).
Edman, P. Pharmaceutical formulations — suspensions and solutions. J. Aerosol Med. 7, S3–S6 (1994).
Patel, A., Cholkar, K., Agrahari, V. & Mitra, A. K. Ocular drug delivery systems: an overview. World J. Pharmacol. 2, 47–64 (2013).
Patel, V. R. & Agrawal, Y. K. Nanosuspension: an approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2, 81–87 (2011).
Owen, A. & Rannard, S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: insights for applications in HIV therapy. Adv. Drug Deliv. Rev. 103, 144–156 (2016).
van Hoogevest, P., Liu, X. & Fahr, A. Drug delivery strategies for poorly water-soluble drugs: the industrial perspective. Expert Opin. Drug Deliv. 8, 1481–1500 (2011).
Rogueda, P. Novel hydrofluoroalkane suspension formulations for respiratory drug delivery. Expert Opin. Drug Deliv. 2, 625–638 (2005).
Rabinow, B. E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 3, 785–796 (2004).
Moghimipour, E., Salimi, A., Rezaee, S., Balack, M. & Handali, S. Influence of flocculating agents and structural vehicles on the physical stability and rheological behavior of nitrofurantoin suspension. Jundishapur J. Nat. Pharm. Prod. 9, e12716 (2014).
Wu, J. P. & Pickle, S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception 89, 495–503 (2014).
Anderson, J. M. & Shive, M. S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 64, 72–82 (2012).
Zong, X. et al. Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofiber membrane. Biomacromolecules 4, 416–423 (2003).
Pan, P. et al. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(L-lactic acid)/poly(d-lactic acid) racemic blends: molecular weight effects. J. Phys. Chem. B 119, 6462–6470 (2015).
Dash, T. K. & Konkimalla, V. B. Poly-ϵ-caprolactone based formulations for drug delivery and tissue engineering: a review. J. Control. Release 158, 15–33 (2012).
Gopferich, A. & Tessmar, J. Polyanhydride degradation and erosion. Adv. Drug Deliv. Rev. 54, 911–931 (2002).
Domb, A. J. et al. in Polymeric Biomaterials (ed. Dumitriu, S.) 91–122 (Marcel Dekker, 2001).
van de Weert, M., Hennink, W. E. & Jiskoot, W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 17, 1159–1167 (2000).
Estey, T., Kang, J., Schwendeman, S. P. & Carpenter, J. F. BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J. Pharm. Sci. 95, 1626–1639 (2006).
Pillai, O. & Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 5, 447–451 (2001).
Goldenberg, I. S. Catgut, silk, and silver — the story of surgical sutures. Surgery 46, 908–912 (1959).
Muffly, T. M., Tizzano, A. P. & Walters, M. D. The history and evolution of sutures in pelvic surgery. J. R. Soc. Med. 104, 107–112 (2011).
Kamaly, N., Yameen, B., Wu, J. & Farokhzad, O. C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116, 2602–2663 (2016).
Vroman, I. & Tighzert, L. Biodegradable polymers. Materials 2, 307–344 (2009).
US Food and Drug Administration. Scenesse. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=210797 (2019).
Hatefi, A. & Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Control. Release 80, 9–28 (2002).
Thakur, R. R., McMillan, H. L. & Jones, D. S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 176, 8–23 (2014).
Kempe, S. & Mader, K. In situ forming implants — an attractive formulation principle for parenteral depot formulations. J. Control. Release 161, 668–679 (2012).
Packhaeuser, C. B., Schnieders, J., Oster, C. G. & Kissel, T. In situ forming parenteral drug delivery systems: an overview. Eur. J. Pharm. Biopharm. 58, 445–455 (2004).
Parent, M. et al. PLGA in situ implants formed by phase inversion: critical physicochemical parameters to modulate drug release. J. Control. Release 172, 292–304 (2013).
Perez-Marreno, R. et al. A six-month, open-label study assessing a new formulation of leuprolide 7.5 mg for suppression of testosterone in patients with prostate cancer. Clin. Ther. 24, 1902–1914 (2002).
Lee, B. K., Yun, Y. & Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 107, 176–191 (2016).
Rungseevijitprapa, W. & Bodmeier, R. Injectability of biodegradable in situ forming microparticle systems (ISM). Eur. J. Pharm. Sci. 36, 524–531 (2009).
Kranz, H. & Bodmeier, R. A novel in situ forming drug delivery system for controlled parenteral drug delivery. Int. J. Pharm. 332, 107–114 (2007).
Wischke, C. & Schwendeman, S. in Fundamentals and Applications of Controlled Release Drug Delivery (eds Siepmann, J., Siegel, R. A. & Rathbone. M. J.) 171–228 (Springer, 2012).
Graham, P. D., Brodbeck, K. J. & McHugh, A. J. Phase inversion dynamics of PLGA solutions related to drug delivery. J. Control. Release 58, 233–245 (1999).
McHugh, A. J. The role of polymer membrane formation in sustained release drug delivery systems. J. Control. Release 109, 211–221 (2005).
Liu, H. & Venkatraman, S. S. Cosolvent effects on the drug release and depot swelling in injectable in situ depot-forming systems. J. Pharm. Sci. 101, 1783–1793 (2012).
Luan, X. & Bodmeier, R. In situ forming microparticle system for controlled delivery of leuprolide acetate: influence of the formulation and processing parameters. Eur. J. Pharm. Sci. 27, 143–149 (2006).
Stewart, S. A., Dominguez-Robles, J., Donnelly, R. F. & Larraneta, E. Implantable polymeric drug delivery devices: classification, manufacture, materials, and clinical applications. Polymers 10, 1379 (2018).
Shastri, V. P. Non-degradable biocompatible polymers in medicine: past, present and future. Curr. Pharm. Biotechnol. 4, 331–337 (2003).
Santos, A. et al. Drug-releasing implants: current progress, challenges and perspectives. J. Mater. Chem. B 2, 6157–6182 (2014).
Silva, G. R. D., Fialho, S. L., Siqueira, R. C., Jorge, R. & Cunha Júnior, A. D. S. Implants as drug delivery devices for the treatment of eye diseases. Braz. J. Pharm. Sci. 46, 585–595 (2010).
Dash, A. K. & Cudworth, G. C. 2nd Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 40, 1–12 (1998).
Fu, Y. & Kao, W. J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 7, 429–444 (2010).
Odom, E. B., Eisenberg, D. L. & Fox, I. K. Difficult removal of subdermal contraceptive implants: a multidisciplinary approach involving a peripheral nerve expert. Contraception 96, 89–95 (2017).
Glasier, A. Implantable contraceptives for women: effectiveness, discontinuation rates, return of fertility, and outcome of pregnancies. Contraception 65, 29–37 (2002).
Mohtashami, Z., Esmaili, Z., Vakilinezhad, M. A., Seyedjafari, E. & Akbari Javar, H. Pharmaceutical implants: classification, limitations and therapeutic applications. Pharm. Dev. Technol. 25, 116–132 (2020).
Danckwerts, M. & Fassihi, A. Implantable controlled release drug delivery systems — a review. Drug Dev. Ind. Pharm. 17, 1465–1502 (1991).
Bourges, J. L. et al. Intraocular implants for extended drug delivery: therapeutic applications. Adv. Drug Deliv. Rev. 58, 1182–1202 (2006).
Martinez-Rus, F., Ferreiroa, A., Ozcan, M., Bartolome, J. F. & Pradies, G. Fracture resistance of crowns cemented on titanium and zirconia implant abutments: a comparison of monolithic versus manually veneered all-ceramic systems. Int. J. Oral Maxillofac. Implants 27, 1448–1455 (2012).
Kluin, O. S., van der Mei, H. C., Busscher, H. J. & Neut, D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin. Drug Deliv. 10, 341–351 (2013).
Yasukawa, T. et al. Drug delivery systems for vitreoretinal diseases. Prog. Retin. Eye Res. 23, 253–281 (2004).
Klemm, K. The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin. Microbiol. Infect. 7, 28–31 (2000).
Al-Jawadi, S., Capasso, P. & Sharma, M. The road to market implantable drug delivery systems: a review on US FDA’s regulatory framework and quality control requirements. Pharm. Dev. Technol. 23, 953–963 (2018).
Keraliya, R. A. et al. Osmotic drug delivery system as a part of modified release dosage form. ISRN Pharm. 2012, 528079 (2012).
Rohloff, C. M. et al. DUROS technology delivers peptides and proteins at consistent rate continuously for 3 to 12 months. J. Diabetes Sci. Technol. 2, 461–467 (2008).
Wright, J. C., Chester, A. E., Skowronski, R. & Lucas, C. Long-term controlled delivery of therapeutic agents via an implantable osmotically driven system: the DUROS implant. Drugs Pharm. Sci. 126, 657–670 (2003).
Wykoff, C. C., Hariprasad, S. M. & Zhou, B. Innovation in neovascular age-related macular degeneration: consideration of brolucizumab, abicipar, and the port delivery system. Opthalmic Surg. Lasers Imaging Retina 49, 913–917 (2018).
Chen, E. R. & Kaiser, P. K. Therapeutic potential of the ranibizumab port delivery system in the treatment of AMD: evidence to date. Clin. Ophthalmol. 14, 1349–1355 (2020).
Renard, E., Costalat, G., Chevassus, H. & Bringer, J. Closed loop insulin delivery using implanted insulin pumps and sensors in type 1 diabetic patients. Diabetes Res. Clin. Pract. 74, S173–S177 (2006).
Elleri, D., Dunger, D. B. & Hovorka, R. Closed-loop insulin delivery for treatment of type 1 diabetes. BMC Med. 9, 120 (2011).
Labelle, M. A., Ispas-Szabo, P., Masseau, I., Chorfi, Y. & Mateescu, M. A. In vivo evaluation of targeted delivery of biological agents using barium sulfate. Int. J. Pharm. 572, 118801 (2019).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 1–17 (2016).
Lin, C. C. & Metters, A. T. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv. Drug Deliv. Rev. 58, 1379–1408 (2006).
Vermonden, T., Censi, R. & Hennink, W. E. Hydrogels for protein delivery. Chem. Rev. 112, 2853–2888 (2012).
Wichterle, O. & Lim, D. Hydrophilic gels for biological use. Nature 185, 117–118 (1960).
Hoare, T. R. & Kohane, D. S. Hydrogels in drug delivery: progress and challenges. Polymer 49, 1993–2007 (2008).
Peppas, N. A. Hydrogels and drug delivery. Curr. Opin. Colloid Interface Sci. 2, 531–537 (1997).
Buwalda, S. J., Vermonden, T. & Hennink, W. E. Hydrogels for therapeutic delivery: current developments and future directions. Biomacromolecules 18, 316–330 (2017).
Caló, E. & Khutoryanskiy, V. V. Biomedical applications of hydrogels: a review of patents and commercial products. Eur. Polym. J. 65, 252–267 (2015).
Ashley, G. W., Henise, J., Reid, R. & Santi, D. V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl Acad. Sci. USA 110, 2318–2323 (2013).
Larraneta, E., Stewart, S., Ervine, M., Al-Kasasbeh, R. & Donnelly, R. F. Hydrogels for hydrophobic drug delivery. classification, synthesis and applications. J. Funct. Biomater. 9, 13 (2018).
Hu, W., Wang, Z., Xiao, Y., Zhang, S. & Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 7, 843–855 (2019).
Nguyen, M. K. & Alsberg, E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog. Polym. Sci. 39, 1236–1265 (2014).
Gibas, I. & Janik, H. Synthetic Polymer Hydrogels for Biomedical Applications (Lviv Polytechnic National Univ., 2010).
Wona, G. & Janik, H. Review: synthetic polymer hydrogels forbiomedical application. Chem. Chem. Technol. 4, 297–304 (2010).
Singh, S. K., Dhyani, A. & Juyal, D. Hydrogel: preparation, characterization and applications. Pharma Innov. 6, 25 (2017).
Ahmed, E. M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 6, 105–121 (2015).
Chun, C., Lee, S. M., Kim, S. Y., Yang, H. K. & Song, S. C. Thermosensitive poly(organophosphazene)-paclitaxel conjugate gels for antitumor applications. Biomaterials 30, 2349–2360 (2009).
Lee, W. Y., Asadujjaman, M. & Jee, J.-P. Long acting injectable formulations: the state of the arts and challenges of poly (lactic-co-glycolic acid) microsphere, hydrogel, organogel and liquid crystal. J. Pharm. Investig. 49, 459–476 (2019).
Natarajan, S. Anti-vascular endothelial growth factor in age-related macular degeneration: puzzle or a silent beginning! Indian J. Ophthalmol. 61, 475–478 (2013).
Stewart, M. W. The study of intravitreal drug pharmacokinetics: does it matter? and if so, how? Expert Opin. Drug Metab. Toxicol. 14, 5–7 (2018).
Li, Y. et al. Pharmacokinetics of Exenatide in nonhuman primates following its administration in the form of sustained-release PT320 and Bydureon. Sci. Rep. 9, 17208 (2019).
Heres, S., Kraemer, S., Bergstrom, R. F. & Detke, H. C. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int. Clin. Psychopharmacol. 29, 299–312 (2014).
Hetrick, E. M. & Schoenfisch, M. H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 35, 780–789 (2006).
Htay, T. & Liu, M. W. Drug-eluting stent: a review and update. Vasc. Health Risk Manag. 1, 263–276 (2005).
US Food and Drug Administration. Resolute Onyx. FDA https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P160043 (2016).
Uurto, I. et al. Drug-eluting biodegradable poly-d/l-lactic acid vascular stents: an experimental pilot study. J. Endovasc. Ther. 12, 371–379 (2005).
Sanchez-Rexach, E., Meaurio, E. & Sarasua, J. R. Recent developments in drug eluting devices with tailored interfacial properties. Adv. Colloid Interfac. 249, 181–191 (2017).
Goldstuck, N. D. Reducing barriers to the use of the intrauterine contraceptive device as a long acting reversible contraceptive. Afr. J. Reprod. Health 18, 15–25 (2014).
Palo, P. Transabdominal and transvaginal ultrasound detection of levonorgestrel IUD in the uterus. Acta Obstet. Gynecol. Scand. 76, 244–247 (1997).
US Food and Drug Administration. Depo-Provera. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020246 (2010).
Shoupe, D. & Mishell, D. R. Norplant: subdermal implant system for long-term contraception. Am. J. Obstet. Gynecol. 160, 1286–1292 (1989).
US Food Drug and Administration. Nexplanon. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021529 (2015).
Crawford, E. D., Moul, J. W., Sartor, O. & Shore, N. D. Extended release, 6-month formulations of leuprolide acetate for the treatment of advanced prostate cancer: achieving testosterone levels below 20 ng/dl. Expert Opin. Drug Metab. Toxicol. 11, 1465–1474 (2015).
US Food and Drug Administration. Lupron Depot. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=019732 (2010).
Zhou, J. et al. Reverse engineering the 1-month Lupron Depot(R). AAPS J. 20, 105 (2018).
Sartor, O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology 61, 25–31 (2003).
US Food and Drug Administration. EligardKit. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021343 (2011).
Wright, J. C. et al. An in vivo/in vitro comparison with a leuprolide osmotic implant for the treatment of prostate cancer. J. Control. Release 75, 1–10 (2001).
US Food and Drug Administration. Viadur. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021088 (2005).
World Health Organization. Global status report on alcohol and health 2018 (WHO, 2018).
Fairbanks, J. et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin. Proc. 95, 1964–1977 (2020).
Azadfard, M., Huecker, M. R. & Leaming, J. M. Opioid Addiction (StatPearls, 2019).
Sinha, R. New findings on biological factors predicting addiction relapse vulnerability. Curr. Psychiat. Rep. 13, 398–405 (2011).
Farid, W. O., Dunlop, S. A., Tait, R. J. & Hulse, G. K. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr. Neuropharmacol. 6, 125–150 (2008).
Neale, J., Tompkins, C. N. E., McDonald, R. & Strang, J. Implants and depot injections for treating opioid dependence: qualitative study of people who use or have used heroin. Drug Alcohol Depend. 189, 1–7 (2018).
Goodbar, N. H. & Hanlon, K. E. Implantable buprenorphine (Probuphine) for maintenance treatment of opioid dependence. Am. Fam. Physician 97, 668–670 (2018).
US Food and Drug Administration. Probuphine. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204442 (2016).
Ling, W., Shoptaw, S. & Goodman-Meza, D. Depot buprenorphine injection in the management of opioid use disorder: from development to implementation. Subst. Abuse Rehabil. 10, 69–78 (2019).
US Food and Drug Administration. Sublocade. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=209819 (2017).
Syed, Y. Y. & Keating, G. M. Extended-release intramuscular naltrexone (VIVITROL): a review of its use in the prevention of relapse to opioid dependence in detoxified patients. CNS Drugs 27, 851–861 (2013).
US Food and Drug Administration. Vivitrol. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process (2010).
Hua, Y. et al. Key factor study for generic long-acting PLGA microspheres based on a reverse engineering of vivitrol. Molecules 26, 1247 (2021).
Sharifi, F., Otte, A., Yoon, G. & Park, K. Continuous in-line homogenization process for scale-up production of naltrexone-loaded PLGA microparticles. J. Control. Release 325, 347–358 (2020).
Gote, V., Sikder, S., Sicotte, J. & Pal, D. Ocular drug delivery: present innovations and future challenges. J. Pharmacol. Exp. Ther. 370, 602–624 (2019).
Chang, J. N. in Handbook of Non-Invasive Drug Delivery Systems (ed. Kulkarni, V. S.) 165–192 (Elsevier, 2010).
Cholkar, K., Vadlapudi, A. D., Trinh, H. M. & Mitra, A. K. in Ocular Pharmacology and Toxicology (ed. Gilger, B. C.) 91–118 (Springer, 2013).
Musch, D. C., Martin, D. F., Gordon, J. F., Davis, M. D., Kuppermann, B. D. & the Ganciclovir Implant Study Group. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. N. Engl. J. Med. 337, 83–90 (1997).
Pearce, W. A., Yeh, S. & Fine, H. F. Management of cytomegalovirus retinitis in HIV and non-HIV patients. Ophthalmic Surg. Lasers Imaging Retina 47, 103–107 (2016).
Choonara, Y. E., Pillay, V., Danckwerts, M. P., Carmichael, T. R. & du Toit, L. C. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J. Pharm. Sci. 99, 2219–2239 (2010).
Stevenson, C. L., Santini, J. T. Jr & Langer, R. Reservoir-based drug delivery systems utilizing microtechnology. Adv. Drug Deliv. Rev. 64, 1590–1602 (2012).
Kane, F. E., Burdan, J., Cutino, A. & Green, K. E. Iluvien (TM): a new sustained delivery technology for posterior eye disease. Expert Opin. Drug Deliv. 5, 1039–1046 (2008).
Wu, X. N. & Lim, L. in Treatment of Non-infectious Uveitis (Lin, P. & Suhler, E.) 157–177 (Springer, 2019).
Lee, S. S., Hughes, P., Ross, A. D. & Robinson, M. R. Biodegradable implants for sustained drug release in the eye. Pharm. Res. 27, 2043–2053 (2010).
US Food and Drug Administration. Durysta. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=211911 (2020).
US Food and Drug Administration. Dextenza. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208742 (2019).
Gibaldi, M. & Levy, G. Pharmacokinetics in clinical practice. I. Concepts. JAMA 235, 1864–1867 (1976).
Wagner, J. G. History of pharmacokinetics. Pharmacol. Ther. 12, 537–562 (1981).
Acknowledgements
Clarivate and PharmaCircle identified, tabulated and partially curated the data about the drug products addressed in this study. We thank D. Bondy for administrative support. This publication is made possible by the generous support of the American people through the US Agency for International Development (USAID) and was prepared under a subaward funded by Family Health International (FHI 360) under cooperative agreement no. AID-OAA-15-00045 funded by USAID. This work was also supported by the Bill and Melinda Gates Foundation (BMGF) through an agreement funded by FHI Partners under grant no. OPP1200867. The content of this publication does not necessarily reflect the views, analysis or policies of FHI 360, FHI Partners, USAID, BMGF or the US Government, nor does any mention of trade names, commercial products or organizations imply endorsement by FHI 360, FHI Partners, USAID, BMGF or the US Government.
Author information
Authors and Affiliations
Contributions
W.L.: Conceptualization, formal analysis, investigation, data curation, writing of the original draft and visualization. J.T.: Formal analysis, investigation and writing of the original draft. T.R.T.: Resources, data curation, writing of the original draft and reviewing and editing. D.L.: Resources, data curation, writing of the original draft and reviewing and editing. S.P.S.: Formal analysis, writing of the original draft, reviewing and editing, and supervision. M.R.P.: Conceptualization, methodology, formal analysis, resources, data curation, writing of the original draft, reviewing and editing, visualization, supervision, project administration and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
M.R.P. is an inventor of patents licensed to companies developing microneedle-based products, is a paid adviser to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products (Micron Biomedical, Clearside Biomedical). This potential conflict of interest has been disclosed and is managed by Georgia Tech. W.L. is an inventor on a pending patent licensed to a company developing microneedle-based products. S.P.S. is an inventor of patents optioned to companies developing long-acting release products and is a paid consultant and scientific adviser/shareholder of companies developing long-acting release products. This potential conflict of interest has been disclosed and is managed by the University of Michigan. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, W., Tang, J., Lee, D. et al. Clinical translation of long-acting drug delivery formulations. Nat Rev Mater 7, 406–420 (2022). https://doi.org/10.1038/s41578-021-00405-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00405-w
This article is cited by
-
Fluorinated GlycoNucleoLipid-based hydrogels as new spatiotemporal stimulable DDS
Drug Delivery and Translational Research (2024)
-
Assembly of peptide nanostructures with controllable sizes
Nano Research (2024)
-
Current State and Opportunities with Long-acting Injectables: Industry Perspectives from the Innovation and Quality Consortium “Long-Acting Injectables” Working Group
Pharmaceutical Research (2023)
-
Red Blood Cell Inspired Strategies for Drug Delivery: Emerging Concepts and New Advances
Pharmaceutical Research (2022)
-
Electrospun Fibers Control Drug Delivery for Tissue Regeneration and Cancer Therapy
Advanced Fiber Materials (2022)