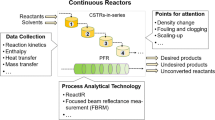
Abstract
Purpose
In pharmaceutical manufacturing, understanding and quantifying how process conditions impact product quality is pivotal to guaranteeing process profitability with sustained product yield. We describe an integrated system model for a commercial-scale continuous manufacturing process of a high-value active pharmaceutical ingredient (API) and its use to optimize process conditions to maximize yield with assured product quality.
Methodology
Global sensitivity analysis (GSA) was used to assess different process parameters’ impacts on API yield in order to guide the selection of decision variables for yield optimization. We then formulated different scenarios for optimization within approved process parameter ranges to propose optimal process conditions to increase API yield.
Results
Within the considered parameter space, varying only key initial starting material and feed reagent mass fractions within their allowed parameter ranges showed potential for + 0.2% yield improvement while varying all process parameters could allow + 0.4% yield improvement.
Conclusions
The general modelling framework to guide control strategies, highlight process improvements in silico, and reduce experimental burden can be applied to multiple pharmaceutical products across different manufacturing modalities and operating modes.







Similar content being viewed by others
Availability of Data
Original datasets and full process details used to construct the system model are not disclosed due to IP constraints. The focus of the study is the application of system modelling to a continuous API manufacturing process. The methodology can be applied to similar processes.
Code Availability
The dynamic flowsheet model is implemented in gPROMS FormulatedProducts v2.0.1 on an Intel® Core™ i-78665 CPU @ 1.90 GHz processor with 16.0 GB of RAM. Codes are not provided due to IP constraints.
Abbreviations
- API:
-
Active pharmaceutical ingredient
- BC:
-
Boundary condition
- CI:
-
Confidence interval
- CPM:
-
Continuous pharmaceutical manufacturing
- CQA:
-
Critical quality attribute
- DAE:
-
Differential algebraic equation
- GSA:
-
Global sensitivity analysis
- HPLC:
-
High-performance liquid chromatography
- HTF:
-
Heat transfer fluid
- KPI:
-
Key performance indicator
- LSA:
-
Local sensitivity analysis
- NLPSQP:
-
Nonlinear sequential quadratic programming
- NMR:
-
Nuclear magnetic resonance spectroscopy
- R&D:
-
Research and development
- RTD:
-
Residence time distribution
- API:
-
Active pharmaceutical ingredient
- BP:
-
By-product
- CO2 :
-
Carbon dioxide
- Imp.:
-
Impurity
- Int.:
-
Intermediate
- SM:
-
Starting material
- -:
-
Latin letters
- A :
-
Heat transfer area [m2]
- Bo :
-
Bodenstein number [–]
- C i :
-
Concentration of species i [mol m−3]
- C i feed :
-
Feed concentration of species i [mol m−3]
- C p :
-
Isobaric specific heat capacity of reaction mixture [J kg−1 K−1]
- C p,HTF :
-
HTF isobaric specific heat capacity [J kg−1 K−1]
- D :
-
Diffusion coefficient [m s−1]
- d R :
-
Reactor diameter [m]
- E a ( j ) :
-
Activation energy of reaction j [J mol−1]
- k j :
-
Rate constant of reaction j [varying units]
- \(k_{0}^{({j)}}\) :
-
Pre-exponential factor of reaction j [varying units]
- K eq ( j ) :
-
Equilibrium constant of reaction j
- L :
-
Length of reactor [m]
- m i :
-
Mass of species i [kg]
- \(\dot{m}\) :
-
Inlet mass flowrate of reaction solution [kg s−1]
- \(\dot{m}\) HTF :
-
HTF mass flowrate [kg s−1]
- N c :
-
Number of components [–]
- N inlet :
-
Number of inlets [–]
- N param :
-
Number of parameters [–]
- N r :
-
Number of reactions [–]
- N s :
-
Number of samples [–]
- N traj :
-
Number of trajectories [–]
- R :
-
Universal gas constant [J mol−1 K−1]
- r j :
-
Rate of reaction j [mol m−3 s−1]
- S T :
-
Sobol’ total sensitivity index [–]
- T :
-
Reaction temperature [K]
- t :
-
Time [s]
- t f :
-
Process duration [s]
- T feed :
-
Temperature of reaction mixture entering reactor [K]
- T HTF :
-
HTF temperature [K]
- T ref :
-
Reaction reference temperature [K]
- U :
-
Overall heat transfer coefficient [W m−2 K−1]
- u :
-
Mean velocity of reaction mixture in reactor [m s−1]
- V :
-
Mixture volume [m3]
- V HT :
-
Mixture volume in the hold-up tank [m3]
- w i in :
-
Mass fraction of component i in inlet stream [–]
- w i out :
-
Mass fraction of component i in outlet stream [–]
- z :
-
Axial position [m]
- -:
-
Greek Letters
- α ij :
-
Order of component i in reaction j [–]
- ΔH r ( j ) :
-
Heat of reaction j [J mol−1]
- θ :
-
Optimization decision variable vector [varying units]
- θ lb :
-
Lower bound on decision variable [varying units]
- θ ub :
-
Upper bound on decision variable [varying units]
- ν ij :
-
Stoichiometric coefficient of component i in reaction j [–]
- ρ HTF :
-
HTF density [kg m−3]
- ρ sol :
-
Reaction mixture density [kg m-3]
References
Hessel V, Kralisch D, Kockmann N, Noël T, Wang Q. Novel process windows for enabling, accelerating, and uplifting flow chemistry. Chemsuschem. 2013;6:746–89.
Plutschack MB, Pieber B, Gilmore K, Seeberger PH. The Hitchhiker’s guide to flow chemistry. Chem Rev. 2017;117:11796–893.
Britton J, Raston CL. Multi-step continuous-flow synthesis. Chem Soc Rev. 2017;52:10159–62.
Cole KP, Reizman BJ, Hess M, Groh JM, Laurila ME, Cope RF, et al. Small-volume continuous manufacturing of merestinib. Part 1. Process Development and Demonstration. Org Process Res Dev. 2019;23:858–69.
Reizman BJ, Cole KP, Hess M, Burt JL, Maloney TD, Johnson MD, et al. Small-volume continuous manufacturing of merestinib. Part 2. Technology transfer and cGMP manufacturing. Org Process Res Dev. 2019;23:870–81.
Jolliffe HG, Gerogiorgis DI. Plantwide design and economic evaluation of two continuous pharmaceutical manufacturing (CPM) cases: Ibuprofen and artemisinin. Comput Chem Eng. 2016;91:269–88.
Bana P, Örkényi R, Lövei K, Lakó Á, Túrós GI, Éles J, et al. The route from problem to solution in multistep continuous flow synthesis of pharmaceutical compounds. Bioorg Med Chem. 2017;25:6180–9.
Costandy JG, Edgar TF, Baldea M. Switching from batch to continuous reactors is a trajectory optimization problem. Ind Eng Chem Res. 2019;58:13718–36.
Teoh SK, Rathi C, Sharratt P. Practical assessment methodology for converting fine chemicals processes from batch to continuous. Org Process Res Dev. 2015;20:414–31.
McMullen JP, Jensen KF. Rapid determination of reaction kinetics with an automated microfluidic system. Org Process Res Dev. 2011;15:398–407.
Grom M, Stavber G, Drnovšek P, Likozar B. Modelling chemical kinetics of a complex reaction network of active pharmaceutical ingredient (API) synthesis with process optimization for benzazepine heterocyclic compound. Chem Eng J. 2016;283:703–16.
Nunn C, DiPietro A, Hodnett N, Sun P, Wells KM. High-throughput automated design of experiment (DoE) and kinetic modeling to aid in process development of an API. Org Process Res Dev. 2018;22:54–61.
Changi SM, Wong S-W. Kinetics model for designing grignard reactions in batch or flow operations. Org Process Res Dev. 2016;20:525–39.
Hone CA, Holmes N, Akien GR, Bourne RA, Muller FL. Rapid multistep kinetic model generation from transient flow data. React Chem Eng. 2017;2:103–8.
Wang K, Han L, Mustakis J, Li B, Magano J, Damon DB, et al. Kinetic and data-driven reaction analysis for pharmaceutical process development. Ind Eng Chem Res. 2020;59:2409–21.
Diab S, Gerogiorgis DI. Technoeconomic mixed integer nonlinear programming (MINLP) optimization for design of liquid-liquid extraction (LLE) cascades in continuous pharmaceutical manufacturing of atropine. AIChE J. 2019;65.
Tang K, Wang Y, Zhang P, Huang Y, Dai G. Process optimization of continuous liquid–liquid extraction in centrifugal contactor separators for separation of oxybutynin enantiomers. Sep Purif Technol. 2015;150:170–8.
Schmidt A, Sixt M, Huter MJ, Mestmäcker F, Strube J. Systematic and model-assisted process design for the extraction and purification of artemisinin from Artemisia annua L.—Part II: Model-based design of agitated and packed columns for multistage extraction and scrubbing. Processes 2018;6:180.
Taifan GSP, Maravelias CT. Integration of graphical approaches into optimization-based design of multistage liquid extraction. Comput Chem Eng. 2020;143:107126.
Vetter T, Burcham CL, Doherty MF. Regions of attainable particle sizes in continuous and batch crystallization processes. Chem Eng Sci. 2014;106:167–80.
Su Q, Nagy ZK, Rielly CD. Pharmaceutical crystallisation processes from batch to continuous operation using MSMPR stages: Modelling, design, and control. Chem Eng Process Process Intensif. 2015;89:41–53.
Diab S, Gerogiorgis DI. No more than three: technoeconomic mixed integer nonlinear programming optimization of mixed suspension, mixed product removal crystallizer cascades for melitracen, an antidepressant API. Ind Eng Chem Res. 2020;59:21458–75.
Wood B, Girard KP, Polster CS, Croker DM. Progress to date in the design and operation of continuous crystallization processes for pharmaceutical applications. Org Process Res Dev. 2019;23:122–44.
Li J, Lai TC, Trout BL, Myerson AS. Continuous crystallization of cyclosporine: The effect of operating conditions on yield and purity. Cryst Growth Des. 2017;17:1000–7.
Lee JW, Horváth Z, O’Brien AG, Seeberger PH, Seidel-Morgenstern A. Design and optimization of coupling a continuously operated reactor with simulated moving bed chromatography. Chem Eng J. 2014;251:355–70.
Gedicke K, Beckmann W, Brandt A, Sapoundjiev D, Lorenz H, Budde U, et al. Coupling chromatography and crystallization for efficient separations of isomers. Adsorption. 2005;11:591–6.
Mestmäcker F, Schmidt A, Huter M, Sixt M, Strube J. Systematic and model-assisted process design for the extraction and purification of artemisinin from Artemisia annua L.—Part III: Chromatographic Purification. Process. 2018.
Lee BW, Peterson JJ, Yin K, Stockdale GS, Liu YC, O’Brien A. System model development and computer experiments for continuous API manufacturing. Chem Eng Res Des. 2020;156:495–506.
Brueggemeier SB, Reiff EA, Lyngberg OK, Hobson LA, Tabora JE. Modeling-based approach towards quality by design for the ibipinabant API step. Org Process Res Dev. 2012;16:567–76.
Dumarey M, Hermanto M, Airiau C, Shapland P, Robinson H, Hamilton P, et al. Advances in continuous active pharmaceutical ingredient (API) manufacturing: real-time monitoring using multivariate tools. J Pharm Innov. 2018;1–14.
Vogel AI, Furniss BS. Experimental techniques. In: Vogel AI, Furniss BS, editors. Vogel’s textbook of practical organic chemistry. Longman, London, UK; 1978.
Process Systems Enterprise 1997–2020. gPROMS. https://psenterprise.com/products/gproms.
Saltelli A, Ratto M, Tarantola S, Campolongo F. Sensitivity analysis for chemical models. Chem Rev. 2005;105:2811–28.
Sobol IM. Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math Comput Simul. 2001;55:271–80.
Boggs PT, Tolle JW. Sequential quadratic programming for large-scale nonlinear optimization. J Comput Appl Math. 2000;124:123–37.
Acknowledgements
The authors would like to thank the following individuals at GSK involved in different aspects of the process’ development and guidance in the system model’s requirements. Project: Peter Shapland, Hannah Robinson, Malcolm Berry, Flavien Susanne, Irene Areri, Peter Clements, Chris Clarke, Peter Hamilton. Commercial Plant Operation: Lesley Wong, Clarence Wong, Gary Breen, Kah Kah Toh, Eneritz Fernandez-Puertas, Krishna Gudena, Stephanie Ng, Hui Ren Seah, Rui Xian Lim. Kinetics: Mark Hughes, Andrew Richards, Augustine Ochen, Sabri Ukuser, Simon Watson.
Funding
The study was funded by a strategic Digital Design capability project at GlaxoSmithKline (GSK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diab, S., Bano, G., Christodoulou, C. et al. Application of a System Model for Continuous Manufacturing of an Active Pharmaceutical Ingredient in an Industrial Environment. J Pharm Innov 17, 1333–1346 (2022). https://doi.org/10.1007/s12247-021-09609-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09609-7