Abstract
Background
WHO acclaims superiority of levofloxacin for the treatment of drug-resistant tuberculosis.
Purpose
The purpose of this research was to develop levofloxacin-loaded solid lipid nanoparticles (LEV-SLN) for use in tuberculosis treatment. The goal was to make LEV-SLN with a mean particle size of less than 300 nm, a drug release duration of more than 12 h, and an LEV-SLN MMAD of less than 5 µm.
Methods
LEV-SLN was made using a single emulsification process, followed by solvent evaporation and lyophilization. A Plackett–Burman screening design and a 32 full factorial design were employed sequentially to explore the impact of various formulation and process parameters on mean particle size, % entrapment efficiency, and in vitro drug release.
Results
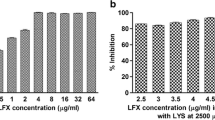
According to the Pareto chart from the Plackett–Burman screening design, the amount of poloxamer and homogenization speed had a significant influence on the mean particle size (p < 0.05). LEV-SLN had a minimum inhibitory concentration of 0.7 µg/ml, whereas pure drug had a minimum inhibitory value of 1.0 µg/ml. The F-ratio for each model developed was higher than the theoretical value (p < 0.05) in a follow-up analysis employing 32 full factorial design, showing that each model was significant.
Conclusion
The best formulation had a mean particle size of 79.70 nm, lasted 12 h in simulated lung fluid, and had an MMAD of 3.71 µm, confirming that the drug may reach up to deep lungs. In the future, preclinical study is needed to develop a realistic dosing regimen and determine the pharmacokinetics and pharmacodynamics of LEV-SLN.
Graphical abstract






Similar content being viewed by others
References
Paliwal R, Paliwal SR, Kenwat R. Kurmi B Das, Sahu MK. Solid lipid nanoparticles: a review on recent perspectives and patents. 2020;30:179–94.
Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery. pharmacological and biopharmaceutical aspects. 2020;7.
Shah S, Bhanderi B, Soniwala M, Chavda J. Lutein-loaded solid lipid nanoparticles for ocular delivery: statistical optimization and ex vivo evaluation. 2021.
Weber S, Zimmer A, Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. 2014;86:7–22.
Subramaniam B, Siddik ZH, Nagoor NH. Optimization of nanostructured lipid carriers: understanding the types, designs, and parameters in the process of formulations. 2020;22:141.
World Health Organisation. Global tuberculosis report. 2020.
Tan ZM, Lai GP, Pandey M, Srichana T, Pichika MR, Gorain B, et al. Novel approaches for the treatment of pulmonary tuberculosis. 2020;12:1196.
Nabi B, Rehman S, Aggarwal S, Baboota S, Ali J. Nano-based anti-tubercular drug delivery: an emerging paradigm for improved therapeutic intervention. 2020;10:1111–21.
Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. 2019;200:e93-142.
Lalloo UG, Ambaram A. New antituberculous drugs in development. 2010;7:143–51.
Gopalan RC, Najafzadeh M, Mohammad MA, Anderson D, Paradkar A, et al. Development and evaluation of nanoemulsion and microsuspension formulations of curcuminoids for lung delivery with a novel approach to understanding the aerosol performance of nanoparticles. 2019;557:254–63.
Huang Z, Huang Y, Wang W, Fu F, Wang W, Dang S, et al. Relationship between particle size and lung retention time of intact solid lipid nanoparticle suspensions after pulmonary delivery. 2020;325:206–22.
Pandey R, Khuller GK. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. 2005;85:227–34.
Anderson CF, Grimmett ME, Domalewski CJ, Cui H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. 2020;12.
Jadhav M, Khan T, Bhavsar C, Momin M, Omri A. Novel therapeutic approaches for targeting TB and HIV reservoirs prevailing in lungs. 2019;16:687–99.
Nemati E, Mokhtarzadeh A, Panahi-Azar V, Mohammadi A, Hamishehkar H, Mesgari-Abbasi M, et al. Ethambutol-loaded solid lipid nanoparticles as dry powder inhalable formulation for tuberculosis therapy. 2019;20:120.
Lee SFK, Laughon BE, McHugh TD, Lipman M. New drugs to treat difficult tuberculous and nontuberculous mycobacterial pulmonary disease. 2019;25:271–80.
Silva DR, Dalcolmo M, Tiberi S, Arbex MA, Munoz-Torrico M, Duarte R, et al. New and repurposed drugs to treat multidrug- and extensively drug-resistant tuberculosis. 2018;44:153–60.
Kendall EA, Shrestha S, Cohen T, Nuermberger E, Dooley KE, Gonzalez-Angulo L, et al. Priority-setting for novel drug regimens to treat tuberculosis: an epidemiologic model. 2017;14:9–23.
Shah SR, Prajapati HR, Sheth DB, Gondaliya EM, Vyas AJ, Soniwala MM, et al. Pharmacokinetics and in vivo distribution of optimized PLGA nanoparticles for pulmonary delivery of levofloxacin. 2020;72:1026–37.
Gaspar MC, Grégoire N, Sousa JJS, Pais AACC, Lamarche I, Gobin P, et al. Pulmonary pharmacokinetics of levofloxacin in rats after aerosolization of immediate-release chitosan or sustained-release PLGA microspheres. 2016;93:184–91.
Shah S, Ghetiya R, Soniwala M, Chavda J. Development and optimization of inhalable levofloxacin nanoparticles for the treatment of tuberculosis. 2020;17.
Pooja D, Tunki L, Kulhari H, Reddy BB, Sistla R. Characterization, biorecognitive activity and stability of WGA grafted lipid nanostructures for the controlled delivery of Rifampicin. 2015;193:11–7.
Singh S, Kushwaha AK, Vuddanda PR, Karunanidhi P, Singh SK. Development and evaluation of solid lipid nanoparticles of raloxifene hydrochloride for enhanced bioavailability. 2013.
Shah SR, Parikh RH, Chavda JR, Sheth NR. Application of Plackett-Burman screening design for preparing glibenclamide nanoparticles for dissolution enhancement. 2013;235:405–11.
Shah SR, Parikh RH, Chavda JR, Sheth NR. Glibenclamide nanocrystals for bioavailability enhancement: formulation design, process optimization, and pharmacodynamic evaluation. 2014.
Shah S, Maheshwari H, Soniwala M, Chavda J. Pulmonary delivery of linezolid nanoparticles for treatment of tuberculosis: design, development, and optimization. 2020.
Booysen LL, Kalombo L, Brooks E, Hansen R, Gilliland J, Gruppo V, et al. In vivo/in vitro pharmacokinetic and pharmacodynamic study of spray-dried poly-(dl-lactic-co-glycolic) acid nanoparticles encapsulating rifampicin and isoniazid. 2013;444:10–17.
Rawal T, Kremer L, Halloum I, Butani S. Dry-powder inhaler formulation of rifampicin: an improved targeted delivery system for alveolar tuberculosis. 2017;30:388–98.
Rawal T, Parmar R, Tyagi RK, Butani S. Rifampicin loaded chitosan nanoparticle dry powder presents: an improved therapeutic approach for alveolar tuberculosis. 2017;154:321–30.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. 2010;12:263–71.
Zarrintaj P, Ramsey JD, Samadi A, Atoufi Z, Yazdi MK, Ganjali MR, et al. Poloxamer: a versatile tri-block copolymer for biomedical applications. 2020;110: 37–67.
Torcello-Gómez A, Wulff-Pérez M, Gálvez-Ruiz MJ, Martín-Rodríguez A, Cabrerizo-Vílchez M, Maldonado-Valderrama J. Block copolymers at interfaces: interactions with physiological media. 2014;206:414–27.
Pandey S, Patel P, Gupta A. Novel solid lipid nanocarrier of glibenclamide: a factorial design approach with response surface methodology. 2018;24:1811–20.
Souto EB, Doktorovova S, Zielinska A, Silva AM. Key production parameters for the development of solid lipid nanoparticles by high shear homogenization. 2019;24:1181–5.
Gupta S, Kesarla R, Chotai N, Misra A, Omri A. Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. 2017;2017:1–18.
U.S. Food and Drug Administration. Levaquin. 2008:1–65.
Helgason T, Awad TS, Kristbergsson K, McClements DJ, Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). 2009;334:75–81.
Chokshi NV, Khatri HN, Patel MM. Formulation, optimization, and characterization of rifampicin-loaded solid lipid nanoparticles for the treatment of tuberculosis. 2018;44:1975–89.
Chokshi NV, Rawal S, Solanki D, Gajjar S, Bora V, Patel BM, et al. Fabrication and characterization of surface engineered rifampicin loaded lipid nanoparticulate systems for the potential treatment of tuberculosis: an in vitro and in vivo evaluation. 2021;110:2221–32.
Acknowledgements
The authors are thankful to Baroque Pharmaceuticals for gift sample of levofloxacin and GUJCOST for providing research grant
Funding
Gujarat Council of Science and Technology (GUJCOST), Gandhinagar, provided a Minor Research Grant titled “Application of nanotechnology for treatment of Tuberculosis” via letter no. GUJCOST/MRP/2016–17/429.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
NA.
Consent for Publication
All the authors have consented for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, S., Shah, N., Amin, S. et al. Studies in Development and Statistical Optimization of Levofloxacin Solid Lipid Nanoparticles for the Treatment of Tuberculosis. J Pharm Innov 17, 1322–1332 (2022). https://doi.org/10.1007/s12247-022-09617-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09617-1