Abstract
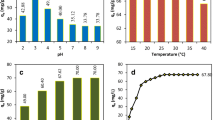
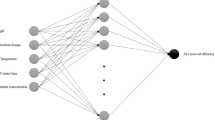
Almond peel waste was collected, characterized, and then used as an adsorbent for removing malachite green (MG) dye from aqueous solutions. The environmental conditions of MG dye adsorption were pH, 2.5–10.5; almond peel dose, 0.25–1.5 g/L; initial MG concentration, 10–60 mg/L; and adsorption time, 0–180 min. These were optimized by using an artificial neural network (ANN) tool. At pH = 8.5, 96.1% of the MG dye could be removed using almond peels. According to the coefficient of determination results, the Langmuir isotherm proved to be the equation that best fit the data of the isotherm study. Furthermore, the kinetic study showed that the data on MG adsorption of the almond peel waste was consistent with the pseudo-second-order model. The ANN model was developed by using a three-layer, feed-forward network with an optimum architecture of 4:10:1. Sigmoid functions were employed in both inputs and hidden layers, as also those hidden in the output layers. The results indicated a high correlation value (R = 0.976) to predict the entire experimental dataset, which indicated the applicability of the ANN tool, to describe the MG adsorption data in a highly accurate manner. The important conclusion of this study, after comparison with other similar adsorbents used in the adsorption process of dye wastewater, revealed that almond peel waste is a cheap, recyclable, and effective adsorptive agent, thus a good alternative to remove dyes from aqueous solutions.
Graphical abstract









Similar content being viewed by others
Data availability
Not applicable.
References
Rasalingam S, Peng R, Koodali RT (2014) Removal of hazardous pollutants from wastewaters: applications of TiO2-SiO2 mixed oxide materials. Journal of Nanomaterials 2014
Zaharia C, Suteu D (2012) Textile organic dyes characteristics, polluting effects and separation/elimination procedures from industrial effluents a critical overview. In. https://doi.org/10.5772/32373
Samchetshabam G, Hussan A, Gon Choudhury T, Gita S, Soholars P, Hussan A (2017) Impact of textile dyes waste on aquatic environments and its treatment.
Ventura-Camargo B, Marin-Morales M (2013) Azo Dyes: characterization and toxicity– a review. Textiles and Light Industrial Science and Technology 2
Raval NP, Shah PU, Shah NK (2017) Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl Water Sci 7(7):3407–3445
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66(3):319–329
Fahmy A, El-Zomrawy A, Saeed AM, Sayed AZ, El-Arab MAE, Shehata H, Friedrich J (2020) Degradation of organic dye using plasma discharge: optimization, pH and energy. Plasma Research Express 2 (1):015009
Ben Arfi R, Karoui S, Mougin K, Ghorbal A (2017) Adsorptive removal of cationic and anionic dyes from aqueous solution by utilizing almond shell as bioadsorbent. Euro-Mediterranean Journal for Environmental Integration 2(1):20. https://doi.org/10.1007/s41207-017-0032-y
Arfi RB, Karoui S, Mougin K, Ghorbal A (2017) Tunisian almond shell for efficient removal of eriochrome black T and malachite green dyes from aqueous solution. Euro-Mediterranean Conference for Environmental Integration. Springer, pp 1383–1385
Rauf M, Ashraf S (2008) Radiation induced degradation of dyes-an overview. J Hazard Mater 166:6–16. https://doi.org/10.1016/j.jhazmat.2008.11.043
Hassaan MA, El Nemr A, Madkour FF (2017) Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. The Egyptian Journal of Aquatic Research 43(1):11–19. https://doi.org/10.1016/j.ejar.2016.09.006
Deng F, Luo X-B, Ding L, Luo S-L (2019) 5 - Application of nanomaterials and nanotechnology in the reutilization of metal ion from wastewater. In: Luo X, Deng F (eds) Nanomaterials for the Removal of Pollutants and Resource Reutilization. Elsevier, pp 149–178. https://doi.org/10.1016/B978-0-12-814837-2.00005-6
Lv, S.H., 7 - High-performance superplasticizer based on chitosan, in Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials, F. Pacheco-Torgal, et al., Editors. 2016, Woodhead Publishing. p. 131–150.
Yu M, Han Y, Li J, Wang L (2017) CO2-activated porous carbon derived from cattail biomass for removal of malachite green dye and application as supercapacitors. Chem Eng J 317:493–502. https://doi.org/10.1016/j.cej.2017.02.105
Mohamad M, Mohammad R, May T, Wei L (2019) Removal of malachite green by sugarcane bagasse biochar using response surface methodology, vol 2068. https://doi.org/10.1063/1.5089328
Kannan C, Sundaram T, Palvannan T (2008) Environmentally stable adsorbent of tetrahedral silica and non-tetrahedral alumina for removal and recovery of malachite green dye from aqueous solution. J Hazard Mater 157(1):137–145
Yin Y, Li C, Song C, Tao P, Sun M, Pan Z, Wang T, Shao M (2016) The design of coal-based carbon membrane coupled with the electric field and its application on the treatment of malachite green (MG) aqueous solution. Colloids Surf, A 506:629–636. https://doi.org/10.1016/j.colsurfa.2016.07.038
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209–210:218–225. https://doi.org/10.1016/j.jhazmat.2012.01.010
Bulut E, Özacar M, Şengil İA (2008) Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microporous Mesoporous Mater 115(3):234–246. https://doi.org/10.1016/j.micromeso.2008.01.039
Ani JU, Akpomie KG, Okoro UC, Aneke LE, Onukwuli OD, Ujam OT (2020) Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl Water Sci 10(2):69. https://doi.org/10.1007/s13201-020-1149-8
Bello OS, Owojuyigbe ES, Babatunde MA, Folaranmi FE (2017) Sustainable conversion of agro-wastes into useful adsorbents. Appl Water Sci 7(7):3561–3571. https://doi.org/10.1007/s13201-016-0494-0
Sorkheh K, Shiran B, Rouhi V, Asadi E, Jahanbazi H, Moradi H, Gradziel T, Martinez-Gomez P (2009) Phenotypic diversity within native Iranian almond (Prunus spp.) species and their breeding potential. Genet Resour Crop Evol 56:947–961. https://doi.org/10.1007/s10722-009-9413-7
Rahemi A The development of almond orchards in Iran. In: III International Symposium on Pistachios and Almonds 591, 2001. pp 177–180
Pirayesh H, Khazaeian A (2012) Using almond ( Prunus amygdalus L.) shell as a bio-waste resource in wood based composite. Composites Part B-engineering - COMPOS PART B-ENG 43. https://doi.org/10.1016/j.compositesb.2011.06.008
Ghaedi M, Zeinali N, Ghaedi AM, Teimuori M, Tashkhourian J (2014) Artificial neural network-genetic algorithm based optimization for the adsorption of methylene blue and brilliant green from aqueous solution by graphite oxide nanoparticle. Spectrochim Acta Part A Mol Biomol Spectrosc 125:264–277. https://doi.org/10.1016/j.saa.2013.12.082
Anupam K, Dutta S, Bhattacharjee C, Datta S (2016) Artificial neural network modelling for removal of chromium (VI) from wastewater using physisorption onto powdered activated carbon 57(8): 3632–3641.
Arghavan FS, Al-Musawi TJ, Allahyari E, Moslehi MH, Nasseh N, Hossein Panahi A (2021) Complete degradation of tamoxifen using FeNi3@SiO2@ZnO as a photocatalyst with UV light irradiation: a study on the degradation process and sensitivity analysis using ANN tool. Mater Sci Semicond Process 128:105725. https://doi.org/10.1016/j.mssp.2021.105725
Ozdes D, Gundogdu A, Duran C, Senturk HB (2010) Evaluation of adsorption characteristics of malachite green onto almond shell (Prunus dulcis). Sep Sci Technol 45(14):2076–2085. https://doi.org/10.1080/01496395.2010.504479
Al-Musawi TJ, Mahvi AH, Khatibi AD, Balarak D (2021) Effective adsorption of ciprofloxacin antibiotic using powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles. Journal of Porous Materialshttps://doi.org/10.1007/s10934-021-01039-7
Alwared AI, Al-Musawi TJ, Muhaisn LF, Mohammed AA (2021) The biosorption of reactive red dye onto orange peel waste: a study on the isotherm and kinetic processes and sensitivity analysis using the artificial neural network approach. Environ Sci Pollut Res 28(3):2848–2859. https://doi.org/10.1007/s11356-020-10613-6
Nasseh N, Al-Musawi TJ, Khosravi R, Hossein Panahi A, Arghavan FS, Barikbin B (2021) FeNi3@SiO2@CuS magnetic nanocomposite: synthesizing, characterization, and application for methylene blue adsorption. Desalin Water Treat 210:402–414
Pauletto P, Gonçalves J, Pinto L, Dotto G, Salau N (2020) Single and competitive dye adsorption onto chitosan–based hybrid hydrogels using artificial neural network modeling. J Colloid Interface Sci 560:722–729
Yahya MD, Abubakar H, Obayomi KS, Iyaka YA, Suleiman B (2020) Simultaneous and continuous biosorption of Cr and Cu (II) ions from industrial tannery effluent using almond shell in a fixed bed column. Results in Engineering 6:100113. https://doi.org/10.1016/j.rineng.2020.100113
Li X, Liu Y, Hao J, Wang W (2018) Study of almond shell characteristics Materials (Basel) 11(9):1782. https://doi.org/10.3390/ma11091782
Li X, Liu Y, Hao J, Wang W (2018) Study of almond shell characteristics Materials 11(9):1782
Trivedi, M., et al., Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. 2015.
Othmani A, Kesraoui A, Boada R, Seffen M, Valiente M (2019) Textile wastewater purification using an elaborated biosorbent hybrid material (luffa–cylindrica–zinc oxide) assisted by alternating current. Water 11 (7). https://doi.org/10.3390/w11071326
Farnane M, Machrouhi A, Elhalil A, Abdennouri M, Qourzal S, Tounsadi H, Barka N (2018) New sustainable biosorbent based on recycled deoiled carob seeds: optimization of heavy metals remediation. J Chem 2018:5748493. https://doi.org/10.1155/2018/5748493
Uddin MT, Rahman MA, Rukanuzzaman M, Islam MA (2017) A potential low cost adsorbent for the removal of cationic dyes from aqueous solutions. Appl Water Sci 7(6):2831–2842. https://doi.org/10.1007/s13201-017-0542-4
Lee S-L, Park J-H, Kim S-H, Kang S-W, Cho J-S, Jeon J-R, Lee Y-B, Seo D-C (2019) Sorption behavior of malachite green onto pristine lignin to evaluate the possibility as a dye adsorbent by lignin. Applied Biological Chemistry 62(1):37. https://doi.org/10.1186/s13765-019-0444-2
Ibrahim SM, Badawy AA, Essawy HA (2019) Improvement of dyes removal from aqueous solution by nanosized cobalt ferrite treated with humic acid during coprecipitation. Journal of Nanostructure in Chemistry 9(4):281–298. https://doi.org/10.1007/s40097-019-00318-9
Uma Y, Sharma U (2013) Removal of malachite green from aqueous solutions by adsorption on to timber waste 4:631–638
Belhachemi M, Addoun F (2011) Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl Water Sci 1(3):111–117. https://doi.org/10.1007/s13201-011-0014-1
Sartape AS, Mandhare AM, Jadhav VV, Raut PD, Anuse MA, Kolekar SS (2017) Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab J Chem 10:S3229–S3238. https://doi.org/10.1016/j.arabjc.2013.12.019
Wang C-J, Li Z, Jiang W-T (2011) Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals. Appl Clay Sci 53(4):723–728. https://doi.org/10.1016/j.clay.2011.06.014
Zhang J, Li Y, Zhang C, Jing Y (2008) Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root. J Hazard Mater 150(3):774–782. https://doi.org/10.1016/j.jhazmat.2007.05.036
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265(1):159–168. https://doi.org/10.1016/j.desal.2010.07.047
Santhi T, Manonmani S, Vasantha VS, Chang YT (2016) A new alternative adsorbent for the removal of cationic dyes from aqueous solution. Arab J Chem 9:S466–S474. https://doi.org/10.1016/j.arabjc.2011.06.004
Acknowledgements
The authors express their sincere gratitude to the Payame Noor University of Tehran, Birjand University of Medical Sciences and Al-Mustaqbal University college for financing and scientific supporting of the current study.
Funding
Birjand University of Medical Sciences has provided financial support for this research.
Author information
Authors and Affiliations
Contributions
N.N., conceptualization, methodology, and project administration; T.A.M., investigation, resources, and data curation; S.F.A., writing—original draft preparation, software, and validation; S.M.A.A and E.A, performed neural network analyzes; T.A.M and A.O., writing—review and editing, resources, and data curation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Musawi, T.J., Arghavan, S.M.A., Allahyari, E. et al. Adsorption of malachite green dye onto almond peel waste: a study focusing on application of the ANN approach for optimization of the effect of environmental parameters. Biomass Conv. Bioref. 13, 12073–12084 (2023). https://doi.org/10.1007/s13399-021-02174-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02174-6