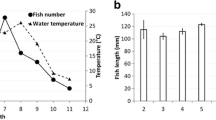
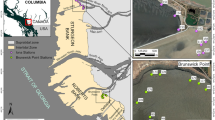
Abstract
Prochilodus lineatus (Valenciennes, 1837) is one of 270 fish species in the Rio Paraná system yet it comprises > 50% of the fish biomass and is the only fish species in the system known to feed entirely on flocculent benthic biofilm. We studied the feeding behavior and diet of P. lineatus, and the composition of benthic biofilm in river channels, the moving littoral, and in isolated floodplain lakes at different stages of the Rio Paraná hydrological cycle. Prochilodus lineatus selectively ingests hydrolysis-labile-organic-matter rich in amino acids while selectively rejecting mineral matter and refractory organic matter. Assessed in terms of g AA assimilated · kJ−1 energy assimilated, the quality of food ingested by P. lineatus ranges from a maintenance level of 5 to 12 mg AA · kJ−1, a level expected to produce near maximum growth. The level of amino acids in benthic biofilm is highest in shallow waters during the advancing flood (the moving littoral), and P. lineatus have been observed to feed in littoral areas so shallow that their dorsal fins and backs are exposed. Feeding intensity increases with river height as the moving littoral expands. Feeding site selection, selective ingestion, and conditional feeding intensity each increase amino acid assimilation and together constitute a trophic strategy that allows P. lineatus to dominate the fish community of the Rio Paraná.






Similar content being viewed by others
Data availability
Data on which this manuscript is based are archived in the Emory University open-access archive Dataverse. Reviewers may access them at https://dataverse.unc.edu/privateurl.xhtml?token=b28d5fa9-0ba2-4946-8506-e57935ebe2e1
Code availability
Does not apply.
References
Agostinho AA, Vazzoler AEAM, Gomes LC, Okada EK (1993) Estratificación espacial y comportamiento de Prochilodus scrofa en distintas fases del ciclo de vida, en la planicie de inundación del alto río Paraná y embalse de Itaipu, Paraná, Brasil. Rev Hydrobiol Trop 26: 79-90
Ahlgren MO (1996) Selective ingestion of detritus by a north temperate omnivorous fish, the juvenile white sucker Catostomus Commersoni. Environ Biol Fish 46(4):375–381
Ahlgren MO, Bowen SH (1992) Comparison of quantitative light microscopy techniques used in diet studies of detritus-consuming omnivores. Hydrobiologia 239(2):79–83. https://doi.org/10.1007/BF00012573
Alldredge AL, Passow U, Logan BE (1993) The abundance and significance of a class of large, transparent organic particles in the ocean. Deep Sea Res Part I 40(6):1131–1140. https://doi.org/10.1016/0967-0637(93)90129-Q
Angelescu V, Gneri FS (1949) Adaptaciones del aparato degestivo al regimen alimenticio en algunos peces del rio Uruguay y del rio de La Plata. I. tipo omnivoro e iliofago en representantes de las familias loricariidae y anostomidea. Rev Inst Nac Invest Cienc Naturales 1: 161-281
Arantes CC, Winemiller KO, Petrere M, Freitas CEC (2019) Spatial variation in aquatic food webs in the Amazon River floodplain. Freshw Sci 38(1):213–228. https://doi.org/10.1086/701841
Bar-Zeev E, Berman-Frank I, Girshevitz O, Berman T (2012) Revised paradigm of aquatic biofilm formation facilitated by microgel transparent exopolymer particles. Proc Natl Acad Sci 109(23):9119–9124. https://doi.org/10.1073/pnas.1203708109
Bayley PB (1973) Studies on the migratory characin, Prochilodus platensis Holmberg 1889, (Pisces, Characoidei) in the River Pilcomayo, South America. J Fish Biol 5(1):25–40. https://doi.org/10.1111/j.1095-8649.1973.tb04428.x
Bayley PB, Castello L, Batista VS, Fabré NN (2018) Response of Prochilodus nigricans to flood pulse variation in the central Amazon. Royal Society Open Science 5(6):1–15. https://doi.org/10.1098/rsos.172232
Blé MC, Arfi R (2009) Seasonal effects on the nutritive value of the natural food of three omnivorous fish (Oreochromis niloticus, Sarotherodon galilaeus, Citharinus citharus) in Batamani Pond (Mali, West Africa)
Bonetto AA (1986) Fish of the Parana system. In: Daves BR, Walker KF (eds) The ecology of river systems. Dr W. Junk Publishers, Dordrecht, pp 573–588
Bonetto AA, Castello HP (1985) Pesca y Piscicultura en Aquas Continentales de America Latina, vol 31. Secretaría General de la Organización de los Estados Americanos, Washington, D.C
Bowen S, Yap MR (2018) Crowding reduces feeding rate, effectiveness of diet selection, and efficiency of digestion by Northern Brook Lamprey ammocetes (Ichthyomyzon fossor). Environ Biol Fishes 101(9):1385–1394. https://doi.org/10.1007/s10641-018-0785-4
Bowen SH (1979a) Determinants of the chemical composition of periphytic detrital aggregate in a tropical lake (Lake Valencia, Venezuela). Archiv for Hydrobiology 87:166–177
Bowen SH (1979b) A nutritional constraint in detritivory by fishes: the stunted population of Sarotherodon mossambicus in Lake Sibaya, South Africa. Ecol Monogr 49:17–31
Bowen SH (1980) Detrital nonprotein amino acids are the key to rapid growth of tilapia in Lake Valencia, Venezuela. Science 207:1216–1218
Bowen SH (1981) Digestion and assimilation of periphytic detrital aggregate by Tilapia mossambica. Trans Am Fish Soc 110(2):239–245. https://doi.org/10.1577/1548-8659(1981)110%3c239:Daaopd%3e2.0.Co;2
Bowen SH (1984) Detrital amino acids and the growth of Sarotherodon mossambicus — a reply to DABROWSKI. Acta Hydrochim Hydrobiol 12(1):55–59. https://doi.org/10.1002/aheh.19840120110
Bowen SH (1987) Composition and nutritional value of detritus. In: Moriarty DJW, Pullin RSV (eds) Detritus and microbial ecology in aquaculture ICLARM Conference Proceedings. vol 14. International Center for Living Aquatic Resources Management, Manila, p 192-216
Bowen SH (1996) Quantitative description of the diet. In: Murphy BR, Willis DW (eds) Fisheries techniques, 2nd edn. American Fisheries Society, Bethesda, pp 513–532
Bowen SH (2021) Digestion and assimilation of benthic biofilm by the Sábalo, Prochilodus lineatus. J Fish Biol: 1-10. https://doi.org/10.1111/jfb.14924
Bowen SH, Bonetto AA, Ahlgren MO (1984) Microorganisms and detritus in the diet of a typical neotropical riverine detritivore, Prochilodus platensis (Pisces: Prochilodontidae). Limnol Oceanogr 29(5):1120–1122. https://doi.org/10.4319/lo.1984.29.5.1120
Bowen SH, Gu B, Huang Z (2006) Diet and digestion in Chinese mud carp Cirrhinus molitorella compared with other ilyophagous fishes. Trans Am Fish Soc 135(5):1383–1388. https://doi.org/10.1577/T05-158.1
Bowen SH, Lutz EV, Ahlgren MO (1995) Dietary protein and energy as determinants of food quality: trophic strategies compared. Ecology 76:899–907
Buddington RK (1980) Hydrolysis-resistant organic matter as a reference for measurement of fish digestive efficiency. Trans Am Fish Soc 109:653–655
Cisternas-Novoa C, Lee C, Engel A (2015) Transparent exopolymer particles (TEP) and Coomassie stainable particles (CSP): differences between their origin and vertical distributions in the ocean. Mar Chem 175:56–71. https://doi.org/10.1016/j.marchem.2015.03.009
Claquin P, Probert I, Lefebvre S, Veron B (2008) Effects of temperature on photosynthetic parameters and TEP production in eight species of marine microalgae. Aquat Microb Ecol 51(1):1–11
Dauda AB, Romano N, Chen WW, Natrah I, Kamarudin MS (2018) Differences in feeding habits influence the growth performance and feeding efficiencies of African catfish (Clarias gariepinus) and lemon fin barb hybrid (Hypsibarbus wetmorei ♂ × Barboides gonionotus ♀) in a glycerol-based biofloc technology system versus a recirculating system. Aquacult Eng 82:31–37. https://doi.org/10.1016/j.aquaeng.2018.06.005
Decho AW (2000) Microbial biofilms in intertidal systems: an overview. Cont Shelf Res 20(10–11):1257–1273
Decho AW, Gutierrez T (2017) Microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol 8(922). https://doi.org/10.3389/fmicb.2017.00922
Dodds WK et al (2014) You are not always what we think you eat: selective assimilation across multiple whole-stream isotopic tracer studies. Ecology 95(10):2757–2767. https://doi.org/10.1890/13-2276.1
Domozych DS, Domozych CR (2008) Desmids and biofilms of freshwater wetlands: development and microarchitecture. Microb Ecol 55(1):81–93. https://doi.org/10.1007/s00248-007-9253-y
Edmondson WT (1974) A simplified method for counting phytplankton. In: Vollenweider RA (ed) A manual on methods for measuring primary production in aquatic environments. IBP Handbook, 2nd edn. Blackwell Scientific Publications, Oxford
Ekasari J et al (2014) The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture 426–427:105–111. https://doi.org/10.1016/j.aquaculture.2014.01.023
Flemming H-C, Neu TR, Wingender J (2016a) The perfect slime: microbial extracellular polymeric substances (EPS). IWA Publishing
Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016b) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Geesey GG, Mutch R, Costerton JW, Green RB (1978) Sessile bacteria: an important component of the microbial population in small mountain streams 1. Limnol Oceanogr 23(6):1214–1223. https://doi.org/10.4319/lo.1978.23.6.1214
Getachew T, Bowen SH, Eyualem A, Zenebe T (2000) Seasonal variations determine diet quality for Oreochromis niloticus L. (Pisces: Cichlidae) in Lake Tana, Ethiopian. SINET: Ethiop J Sci 23(1):13–23
Gomes LC, Agostinho AA (1997) Influence of the flooding regime on the nutritional state and juvenile recruitment of the curimba, Prochilodus scrofa, Steindachner, in upper Paraná River, Brazil. Fish Manage Ecol 4:263–274
González-Bergonzoni I et al (2019) Origin of fish biomass in a diverse subtropical river: an allochthonic-supported biomass increase following flood pulses. Ecosystems 22(8):1736–1753. https://doi.org/10.1007/s10021-019-00370-0
Harper A (1976) Protein and amino acids in the regulation of food intake. In: Novin D, Wyricka W, Bray G (eds) Hunger: basic mechanisms and clinical implications. Raven Press, New York, pp 103–113
Heidman MK, Holley LL, Chambers RM, Sanderson SL (2012) Selective feeding on nutrient-rich particles by gizzard shad Dorosoma cepedianum does not involve mechanical sorting. Aquat Biol 17(2):129–139
Hobbie JE, Lee C (1980) Microbial production of extracellular material: importance in benthic ecology. In: Tenore KR, and Coull, B.C. Tue (ed) Marine benthic dynamics. Conference: 11. Bell W. Baruch symposium in marine science, Georgetown, SC, USA, Apr 1979. U.S Office of Scientific and Technical Information, p 341–346
Hongyue Dang CRL (2016) Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 80(1):91–138
Jiménez A, Elner RW, Favaro C, Rickards K, Ydenberg RC (2015) Intertidal biofilm distribution underpins differential tide-following behavior of two sandpiper species (Calidris mauri and Calidris alpina) during northward migration. Estuar Coast Shelf Sci 155:8–16. https://doi.org/10.1016/j.ecss.2014.12.038
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. In: Dodge DP (ed) Proceedings of the International Large River Symposium Can Spec Publ Fish Aquat Sci. vol 106. Can Spec Publ Fish Aquat Sci, p 110–127
Kaushik NK, Hynes HBN (1968) Experimental study on the role of autumn-shed leaves in aquatic environments. J Ecol 56:229–243
Keitel J, Zak D, Hupfer M (2016) Water level fluctuations in a tropical reservoir: the impact of sediment drying, aquatic macrophyte dieback, and oxygen availability on phosphorus mobilization. Environ Sci Pollut Res 23(7):6883–6894. https://doi.org/10.1007/s11356-015-5915-3
Lasso CA, Machado-Allison A, Taphorn DC (2016) Fishes and aquatic habitats of the Orinoco River Basin: diversity and conservation. J Fish Biol 89(1):174–191. https://doi.org/10.1111/jfb.13010
Lawrence JR, Neu TR, Paule A, Korber DR, Wolfaardt GM (2016) Aquatic biofilms: development, cultivation, analyses, and applications manual of environmental microbiology, 4 th edition. American Society of Microbiology
Lescano de Almeida VL, Kawakami de Resende E, de Sousa Lima M, Ferreira CJA (1993) Dieta e atividade alimentar de Prochilodus lineatus (characiformes, prochilodontidae) no Pantanal do Miranda-Aquidauana, Matto Grosso do Sul, Brasil. Revista Unimar, Maringa 15 (Supplement):125-141
Lourenço PM, Catry T, Lopes RJ, Piersma T, Granadeiro JP (2017) Invisible trophic links? Quantifying the importance of non-standard food sources for key intertidal avian predators in the Eastern Atlantic. Mar Ecol Prog Ser 563:219–232
Lu J, Faggotter SJ, Bunn SE, Burford MA (2017) Macrophyte beds in a subtropical reservoir shifted from a nutrient sink to a source after drying then rewetting. Freshw Biol 62(5):854–867. https://doi.org/10.1111/fwb.12904
Makkar P, Dawra R, Singh B (1988) Determination of both tannin and protein in a tannin-protein complex. J Agric Food Chem 36(3):523–525. https://doi.org/10.1021/jf00081a600
Marchese MR et al (2014) Food webs of the Paraná River floodplain: assessing basal sources using stable carbon and nitrogen isotopes. Limnologica 46:22–30. https://doi.org/10.1016/j.limno.2013.11.004
Mundahl ND, Wissing TE (1988) Selection and digestive efficiencies of gizzard shad feeding on natural detritus and two laboratory diets. Trans Am Fish Soc 117:480–487
Ocock JF, Brandis KJ, Wolfenden BJ, Jenkins KM, Wassens S (2018) Gut content and stable isotope analysis of tadpoles in floodplain wetlands. Aust J Zool 66(4):261–271. https://doi.org/10.1071/ZO18043
Odum WE (1968) The ecological significance of fine particle selection by the striped mullet Mugil cephalus. Limnol Oceanogr 13:92–98
Odum WE, Kirk PW, Zieman JC (1979) Non-protein nitrogen compounds associated with particles of vascular plant detritus. Oikos 32(3):363–367. https://doi.org/10.2307/3544746
Orvain F et al (2014) Tidal and seasonal effects on the short-term temporal patterns of bacteria, microphytobenthos and exopolymers in natural intertidal biofilms (Brouage, France). J Sea Res 92:6–18. https://doi.org/10.1016/j.seares.2014.02.018
Passow U, Alldredge AL (1995) A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP). Limnol Oceanogr 40(7):1326–1335. https://doi.org/10.4319/lo.1995.40.7.1326
Pesoa N, Schulz U (2010) Diel and seasonal movements of grumatã Prochilodus lineatus (Valenciennes 1836) (Characiformes: Prochilodontidae) in the Sinos River, Southern Brazil. Braz J Biol 70:1169–1177
Pisani O, Yamashita Y, Jaffé R (2011) Photo-dissolution of flocculent, detrital material in aquatic environments: contributions to the dissolved organic matter pool. Water Res 45(13):3836–3844. https://doi.org/10.1016/j.watres.2011.04.035
Rice DL (1982) The detritus nitrogen problem: new observations and perspectives from organic geochemistry. Mar Ecol Prog Ser 9(2):153–162
Saldana J, Venables B (1983) Energy compartmentalization in a migratory fish, Prochilodus mariae (Prochilodontidae), of the Orinoco River. Copeia 3:617–623
Samocha TM (2019) Biofloc. In: Samocha TM (ed) Sustainable biofloc systems for marine shrimp. Academic Press, p 29-36
Schönbrunner IM, Preiner S, Hein T (2012) Impact of drying and re-flooding of sediment on phosphorus dynamics of river-floodplain systems. Sci Total Environ 432(10):329–337. https://doi.org/10.1016/j.scitotenv.2012.06.025
Silva EA, Stewart DJ (2017) Reproduction, feeding and migration patterns of Prochilodus nigricans (Characiformes: Prochilodontidae) in northeastern Ecuador. Neotropical Ichthyol 15
Simon KS, Benfield EF, Macko SA (2003) Food web structure and the role of epilithic biofilms in cave streams. Ecology 84(9):2395–2406. https://doi.org/10.1890/02-334
Sutton TM, Bowen SH (1994) Significance of organic detritus in the diet of larval lampreys in the Great Lakes basin. Can J Fish Aquat Sci 51:2380–2387
Sverlij SB, Espinach Ros A, Orti G (1993) Sinopsis de los datos biológicos y pesqueros del sábalo, Prochilodus lineatus (Valenciennes, 1847) FAO Sinopsis sobre la pesca 154, vol 154. Food and Agriculture Organization of the United Nations, Rome
Tansel B (2018) Morphology, composition and aggregation mechanisms of soft bioflocs in marine snow and activated sludge: a comparative review. J Environ Manage 205:231–243. https://doi.org/10.1016/j.jenvman.2017.09.082
Taylor BW, Flecker AS, Hall RO Jr (2006) Loss of a harvested fish species disrupts carbon flow in a diverse tropical river. Science 313:833–836
Yap MR, Bowen SH (2003) Feeding by Northern Brook Lamprey (Ichthyomyzon fossor) on Sestonic biofilm fragments: habitat selection results in ingestion of a higher quality diet. J Great Lakes Res 29(Supplement 1):15–25
Yossa MI, Araujo-Lima CARM (1998) Detritivory in two Amazonian fish species. J Fish Biol 52(6):1141–1153. https://doi.org/10.1111/j.1095-8649.1998.tb00961.x
Acknowledgements
M.O. Ahlgren participated in planning the study, participated in three of the five sampling periods, planned and conducted many of the laboratory analyses, and would have participated in preparation of the manuscript had she not died tragically while serving as a first responder in a marine rescue event. This work was supported by the Conservation, Food, and Health Foundation of Boston, MA, USA, and by generous in-kind support from the Centro de Ecologia Aplicada del Litoral (CECOAL), Corrientes, Argentina, a division of CONICET. Director Juan Josè Neiff’s broad understanding of the Paraná ecosystem and the local knowledge and skill of CECOAL field personnel Sr. Nicholas T. Roberto and Sr. Luis L Benetti were essential to the project’s success. P. Bayley and T. Sutton provided helpful critical reviews of an early draft of this report. Megan Slemons, Emory University GIS Librarian, prepared Fig. 1.
Funding
This work was supported by the Conservation, Food, and Health Foundation of Boston, MA, USA, and by in-kind support from the Centro de Ecologia Aplicada del Litoral (CECOAL), Corrientes, Argentina, a division of CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No approval was required or sought when this research was conducted. All aspects of the research were conducted in compliance with Argentinian law 14346 Article 3 concerning ethical research using vertebrate animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bowen, S.H. The river flood pulse, benthic biofilm, and the nutrition of Prochilodus lineatus. Environ Biol Fish 105, 213–230 (2022). https://doi.org/10.1007/s10641-022-01211-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01211-1