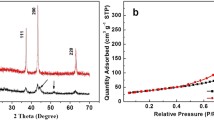
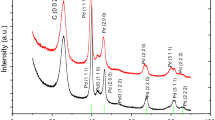
Abstract
Glucose oxidation reaction is kinetically sluggish and demands a highly efficient catalyst. Herein, we report a direct glucose alkaline fuel cell (DGAFC) using physically mixed Ni2Co nanoparticles and reduced graphene oxide (Ni2Co-rGO) supported on nickel foam as an anode to promote glucose oxidation. The DGAFC with the Ni2Co-rGO anode delivered a remarkably enhanced peak power density of 40.44 W/m2 at room temperature, which was 74.91% higher than the control (23.12 W/m2). Different techniques including XPS, SEM, linear sweep voltammetry, and electrochemical impedance spectroscopy were used to explore the physiochemical and electrochemical properties of the catalyst. Our results demonstrate that Ni2Co-rGO exhibits an excellent activity toward glucose oxidation in alkaline medium, leading to a drastic improvement in the anode performance. There should be a synergistic effect between rGO and Ni2Co. In the Ni2Co-rGO nanocomposite, rGO not only acts as a support for the Ni2Co nanoparticles and also promotes the electron transfer during the glucose oxidation process. This study can promote the development of bi-transition metal-based catalysts for DGAFCs.
Graphical abstract










Similar content being viewed by others
References
Wang Y, Diaz DFR, Chen KS, Wang Z, Adroher XC (2020) Materials, technological status, and fundamentals of PEM fuel cells–a review. Materials today 32:178–203
Pan Z, An L, Zhao T, Tang Z (2018) Advances and challenges in alkaline anion exchange membrane fuel cells. Prog Energy Combust Sci 66:141–175
Wang J, Wang H, Fan Y (2018) Techno-economic challenges of fuel cell commercialization. Engineering 4(3):352–360
Wilberforce T, Ijaodola O, Ogungbemi E, Khatib F, Leslie T, El-Hassan Z, Thomposon J, Olabi A (2019) Technical evaluation of proton exchange membrane (PEM) fuel cell performance—a review of the effects of bipolar plates coating. Renewable and Sustainable Energy Reviews. 113:109286
Alias M, Kamarudin S, Zainoodin A, Masdar M (2020) Active direct methanol fuel cell: an overview. Int J Hydrog Energy 45(38):19620–19641
Hao W, Ma H, Sun G, Li Z (2019) Magnesia phosphate cement composite bipolar plates for passive type direct methanol fuel cells. Energy 168:80–87
Zakaria Z, Kamarudin SK (2016) Direct conversion technologies of methane to methanol: an overview. Renew Sustain Energy Rev 65:250–261
Mohammed H, Al-Othman A, Nancarrow P, Tawalbeh M, El Haj AM (2019) Direct hydrocarbon fuel cells: a promising technology for improving energy efficiency. Energy 172:207–219
Leo TJ, Raso MA, Navarro E, Mora E (2013) Long term performance study of a direct methanol fuel cell fed with alcohol blends. Energies 6(1):282–293
Fujiwara N, Yamazaki S-i, Siroma Z, Ioroi T, Senoh H, Yasuda K (2009) Nonenzymatic glucose fuel cells with an anion exchange membrane as an electrolyte. Electrochemistry communications 11(2):390–392
Torigoe K, Takahashi M, Tsuchiya K, Iwabata K, Ichihashi T, Sakaguchi K, Sugawara F, Abe M (2018) High-power abiotic direct glucose fuel cell using a gold–platinum bimetallic anode catalyst. ACS omega 3(12):18323–18333
Lawson K, Rossi R, Regan JM, Logan BE (2020) Impact of cathodic electron acceptor on microbial fuel cell internal resistance. Bioresource technology. 316:123919
Silveira G, de Aquino Neto S, Schneedorf JM (2020) Development, characterization and application of a low-cost single chamber microbial fuel cell based on hydraulic couplers. Energy. 208:118395
Sorrentino I, Gentil S, Nedellec Y, Cosnier S, Piscitelli A, Giardina P, Le Goff A (2019) POXC laccase from pleurotus ostreatus: a high-performance multicopper enzyme for direct oxygen reduction reaction operating in a proton-exchange membrane fuel cell. ChemElectroChem. 6(4):1023–1027
Cracknell JA, Vincent KA, Armstrong FA (2008) Enzymes as working or inspirational electrocatalysts for fuel cells and electrolysis. Chemical Reviews. 108(7):2439–2461
Ahmad YH, Mohamed AT, El-Shafei A, Al-Qaradawi SY, Aljaber AS (2020) Facile one-step synthesis of supportless porous AuPtPd nanocrystals as high performance electrocatalyst for glucose oxidation reaction. International Journal of Hydrogen Energy. 45(38):19163–19173
Xu H, Yan B, Zhang K, Wang J, Li S, Wang C, Du Y, Yang P, Jiang S, Song S (2017) N-doped graphene-supported binary PdBi networks for formic acid oxidation. Appl Surf Sci 416:191–199
Huang J, Zhao P, Jin X, Wang Y, Yuan H, Zhu X (2020) Enzymatic biofuel cells based on protein engineering: recent advances and future prospects. Biomaterials science 8(19):5230–5240
Xiao X, Xia H-q, Wu R, Bai L, Yan L, Magner E, Cosnier S, Lojou E, Zhu Z, Liu A (2019) Tackling the challenges of enzymatic (bio) fuel cells. Chemical reviews 119(16):9509–9558
Irfan M, Liu X, Li S, Khan IU, Li Y, Wang J, Wang X, Du X, Wang G, Zhang P (2020) High-performance glucose fuel cell with bimetallic Ni–Co composite anchored on reduced graphene oxide as anode catalyst. Renewable Energy 155:1118–1126
Frei M, Martin J, Kindler S, Cristiano G, Zengerle R, Kerzenmacher S (2018) Power supply for electronic contact lenses: abiotic glucose fuel cells vs. Mg/Air batteries Journal of Power Sources 401:403–414
Le PG, Kim MI (2021) Research progress and prospects of nanozyme-based glucose biofuel cells. Nanomaterials 11(8):2116
Bahari M, Malmberg MA, Brown DM, Nazari SH, Lewis RS, Watt GD, Harb JN (2020) Oxidation efficiency of glucose using viologen mediators for glucose fuel cell applications with non-precious anodes. Applied Energy 261:114382
Barzi S, Zhiani M, Ahmadi A (2019) Evaluation of carbon supported Fe–Co electrocatalyst for selective oxygen reduction to use in implantable glucose fuel cell. International Journal of Hydrogen Energy 44(59):31515–31524
Basu D, Basu S (2012) Performance studies of Pd–Pt and Pt–Pd–Au catalyst for electro-oxidation of glucose in direct glucose fuel cell. International journal of hydrogen energy 37(5):4678–4684
Rafaïdeen T, Baranton S, Coutanceau C (2019) Highly efficient and selective electrooxidation of glucose and xylose in alkaline medium at carbon supported alloyed PdAu nanocatalysts. Appl Catal B 243:641–656
Escalona-Villalpando R, Gurrola M, Trejo G, Guerra-Balcázar M, Ledesma-García J, Arriaga L (2018) Electrodeposition of gold on oxidized and reduced graphite surfaces and its influence on glucose oxidation. J Electroanal Chem 816:92–98
Gao M, Liu X, Irfan M, Shi J, Wang X, Zhang P (2018) Nickle-cobalt composite catalyst-modified activated carbon anode for direct glucose alkaline fuel cell. International Journal of Hydrogen Energy 43(3):1805–1815
Sarwar E, Noor T, Iqbal N, Mehmood Y, Ahmed S, Mehek R (2018) Effect of Co-Ni ratio in graphene based bimetallic electro-catalyst for methanol oxidation. Fuel Cells 18(2):189–194
Yan Z, Xu Z, Yang Z, Yue L, Huang L (2019) Graphene oxide/Fe2O3 nanoplates supported Pt for enhanced room-temperature oxidation of formaldehyde. Appl Surf Sci 467–468:277–285
Han J, Jun B-M, Heo J, Lee G, Yoon Y, Park CM (2019) Highly efficient organic dye removal from waters by magnetically recoverable La2O2CO3/ZnFe2O4-reduced graphene oxide nanohybrid. Ceramics International 45(15):19247–19256
Sun S, Zhang G, Gauquelin N, Chen N, Zhou J, Yang S, Chen W, Meng X, Geng D, Banis MN (2013) Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Scientific Reports 3(1):1–9
Baronia R, Goel J, Tiwari S, Singh P, Singh D, Singh SP, Singhal SK (2017) Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. International Journal of Hydrogen Energy 42(15):10238–10247
Liu J, Choi HJ, Meng L-Y (2018) A review of approaches for the design of high-performance metal/graphene electrocatalysts for fuel cell applications. J Ind Eng Chem 64:1–15
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. Journal of the american chemical society 80(6):1339–1339
Wang H, Wang G, Ling Y, Qian F, Song Y, Lu X, Chen S, Tong Y, Li Y (2013) High power density microbial fuel cell with flexible 3D graphene–nickel foam as anode. Nanoscale 5(21):10283–10290
Akhavan O, Ghaderi E, Aghayee S, Fereydooni Y, Talebi A (2012) The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. Journal of Materials Chemistry 22(27):13773–13781
Sharma K, Maiti K, Kim NH, Hui D, Lee JH (2018) Green synthesis of glucose-reduced graphene oxide supported Ag-Cu2O nanocomposites for the enhanced visible-light photocatalytic activity. Compos B Eng 138:35–44
Dong F, Liu X, Irfan M, Yang L, Li S, Ding J, Li Y, Khan IU, Zhang P (2019) Macaroon-like FeCo2O4 modified activated carbon anode for enhancing power generation in direct glucose fuel cell. International Journal of Hydrogen Energy 44(16):8178–8187
Yang L, Liu X, Ding J, Li S, Dong F, Irfan M, Li Y, Wang G, Du X, Zhang P (2019) Chlorella-derived porous heteroatom-doped carbons as robust catalysts for oxygen reduction reaction in direct glucose alkaline fuel cell. International Journal of Hydrogen Energy 44(5):2823–2831
Ho J, Li Y, Dai Y, Kim T, Wang J, Ren J, Yun H, Liu X (2021) Ionothermal synthesis of N-doped carbon supported CoMn2O4 nanoparticles as ORR catalyst in direct glucose alkaline fuel cell. International Journal of Hydrogen Energy 46(39):20503–20515
Liu Q, Zhou Q, Gao C, Liu L, Ye H (2021) Excellent electrochemical stability of Co3O4 array with carbon hybridization derived from metal-organic framework. Nanotechnology 32(48):485710
Yang Y-L, Liu X-H, Hao M-Q, Zhang P-P (2015) Performance of a low-cost direct glucose fuel cell with an anion-exchange membrane. International journal of hydrogen energy 40(34):10979–10984
Wang L, Li M, Huang Z, Li Y, Qi S, Yi C, Yang B (2014) Ni–WC/C nanocluster catalysts for urea electrooxidation. J Power Sources 264:282–289
Ding Y, Zhang P, Zhuo Q, Ren H, Yang Z, Jiang Y (2011) A green approach to the synthesis of reduced graphene oxide nanosheets under UV irradiation. Nanotechnology 22(21):215601
Stobinski L, Lesiak B, Malolepszy A, Mazurkiewicz M, Mierzwa B, Zemek J, Jiricek P, Bieloshapka I (2014) Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectrosc Relat Phenom 195:145–154
Ban F, Majid SR, Huang NM, Lim H (2012) Graphene oxide and its electrochemical performance. Int. J. Electrochem. Sci 7(5):4345–4351
Wang T, Zhang S, Yan X, Lyu M, Wang L, Bell J, Wang H (2017) 2-Methylimidazole-derived Ni–Co layered double hydroxide nanosheets as high rate capability and high energy density storage material in hybrid supercapacitors. ACS applied materials & interfaces 9(18):15510–15524
Liang H, Lin J, Jia H, Chen S, Qi J, Cao J, Lin T, Fei W, Feng J (2018) Hierarchical NiCo-LDH/NiCoP@ NiMn-LDH hybrid electrodes on carbon cloth for excellent supercapacitors. Journal of Materials Chemistry A 6(31):15040–15046
Cheng M, Yang R, Zhang L, Shi Z, Yang W, Wang D, Xie G, Shi D, Zhang G (2012) Restoration of graphene from graphene oxide by defect repair. Carbon 50(7):2581–2587
Martin A, Escarpa A (2014) Graphene: the cutting–edge interaction between chemistry and electrochemistry. TrAC, Trends Anal Chem 56:13–26
Tuinstra F, Koenig JL (1970) Raman spectrum of graphite. The Journal of chemical physics 53(3):1126–1130
Farooqui U, Ahmad A, Hamid N (2018) Graphene oxide: a promising membrane material for fuel cells. Renew Sustain Energy Rev 82:714–733
Chen J, Zheng H, Kang J, Yang F, Cao Y, Xiang M (2017) An alkaline direct oxidation glucose fuel cell using three-dimensional structural Au/Ni-foam as catalytic electrodes. RSC Advances 7(5):3035–3042
Lemoine C, Dubois L, Napporn TW, Servat K, Kokoh KB (2020) Electrochemical energy conversion from direct oxidation of glucose on active electrode materials. Electrocatalysis 11(2):170–179
Sakai H, Nakagawa T, Tokita Y, Hatazawa T, Ikeda T, Tsujimura S, Kano K (2009) A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy & Environmental Science 2(1):133–138
Chen J, Zhao CX, Zhi MM, Wang K, Deng L, Xu G (2012) Alkaline direct oxidation glucose fuel cell system using silver/nickel foams as electrodes. Electrochimica acta 66:133–138
Funding
This work was partially supported by the National Key R&D Program of China (Grant No. 2019YFC1407800) and the Natural Science Foundation of Tianjin City (Grant No. 19YFZCSN01130).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• the catalyst has the advantages of low cost
Easy fabrication and high efficiency.
• Maximum power density of 40.44 W/m2 was obtained under ambient conditions.
• A synergistic effect between Ni2Co and graphene was proposed.
• This study can facilitate the development of cost-effective catalysts for DGAFC
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Ding, J., Liu, X. et al. Physically mixed Ni2Co/graphene catalyst for enhanced glucose oxidation in a glucose fuel cell. Biomass Conv. Bioref. 14, 525–537 (2024). https://doi.org/10.1007/s13399-022-02317-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02317-3