Abstract
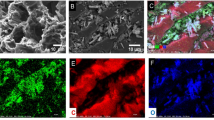
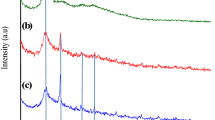
Rapid urbanization has resulted in the discharge of large amounts of polyaromatic hydrocarbons (PAH) into the environment. Due to PAH-associated health hazards, their presence in water bodies has attracted increasing concern worldwide. Hence, it is necessary to device, new, efficient, and cost-effective technologies for their removal. The present work reports the fabrication of a novel chitosan linked activated carbon-nano-bentonite composite (AC-NB-C) membrane removal of acenaphthene (ACE) and naphthalene (NAP) from aqueous solutions containing both PAHs individually as well as a mixture (MP). The formulated membrane was characterized using SEM, TEM, EDS, FT-IR, XRD, and BET. The PAH removal efficiency of the membrane was evaluated in terms of varying process parameters such as initial PAH concentration, pH, and feed pressure. Process optimization was conducted using the central composite design (CCD) feature of response surface methodology (RSM). Reusability potential and antifouling property of the membrane was also evaluated. Under optimized conditions, the AC-NB-C membrane demonstrated a mixed PAH (MP) rejection of approximately 99.3%. The BET surface area and total pore volume of the membrane were observed to be 482.07 m2g–1 and 0.251 cm3g–1 respectively. The FT-IR analysis suggested π-π interaction and H-bonding as possible mechanisms guiding PAH uptake. A 4.9% reduction in flux ratio indicated good antifouling potential of the AC-NB-C membranes. Moreover, the membrane could be regenerated and reused for 7 consecutive cycles of filtration without any decline in its efficiency. Also, the optimum membrane performance recorded at pH 6 established its immense potential for large-scale treatment of municipal wastewater whose pH values are normally between 6 and 8.





Similar content being viewed by others
References
Algarín M, Amaya M, Solano R, Patiño-Ruiz D, Herrera A (2021) Synthesis of a magnetic iron oxide/zinc oxide engineered nanocatalyst for enhanced visible-light photodegradation of Cartasol brilliant violet 5BFN in aqueous solution. Nano-Struct Nano-Objects 26:100730. https://doi.org/10.1016/j.nanoso.2021.100730
Yakout SM, Daifullah AAM, El–Reefy SA, (2013) Adsorption of naphthalene, phenanthrene and pyrene from aqueous solution using low–cost activated carbon derived from agricultural wastes. Adsorp Sci Technol 31(4):293–302. https://doi.org/10.1260/0263-6174.31.4.293
Barman SR, Banerjee P, Mukhopadhayay A, Das P (2017) Biodegradation of acenapthene and naphthalene by Pseudomonas mendocina: process optimization, and toxicity evaluation. J Environ Chem Eng 5(5):4803–4812. https://doi.org/10.1016/j.jece.2017.09.012
Shen G, Wang W, Yang Y, Zhu C, Min Y, Xue M, Ding J, Li W, Wang B, Shen H, Wang R, Wang X, Tao S (2010) Emission factors and particulate matter size distribution of polycyclic aromatic hydrocarbons from residential coal combustions in rural Northern China. Atmos Environ 44(39):5237–5243. https://doi.org/10.1016/j.atmosenv.2010.08.042
Gao B, Yu JZ, Li SX, Ding X, He QF, Wang XM (2011) Roadside and rooftop measurements of polycyclic aromatic hydrocarbons in PM2.5 in urban Guangzhou: evaluation of vehicular and regional combustion source contributions. Atmos Environ 45(39):7184–91. https://doi.org/10.1016/j.atmosenv.2011.09.005
Ahmad J, Naeem S, Ahmad M, Usman AR, Al-Wabel MI (2019) A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J Environ Manage 246:214–228. https://doi.org/10.1016/j.jenvman.2019.05.152
Saad ME, Khiari R, Elaloui E, Moussaoui Y (2014) Adsorption of anthracene using activated carbon and Posidonia oceanica. Arab J Chem 7(1):109–113. https://doi.org/10.1016/j.arabjc.2013.11.002
Nam KJ, Li Q, Heo SK, Tariq S, Loy-Benitez J, Woo TY, Yoo CK (2021) Inter-regional multimedia fate analysis of PAHs and potential risk assessment by integrating deep learning and climate change scenarios. J Hazard Mater 411:125149. https://doi.org/10.1016/j.jhazmat.2021.125149
Barman SR, Banerjee P, Das P, Mukhopadhayay A (2018) Urban wood waste as precursor of activated carbon and its subsequent application for adsorption of polyaromatic hydrocarbons. Int J Energ and Water Res 2(1–4):1–3. https://doi.org/10.1007/s42108-018-0001-4
Cabal B, Budinova T, Ania CO, Tsyntsarski B, Parra JB, Petrova B (2009) Adsorption of naphthalene from aqueous solution on activated carbons obtained from bean pods. J Hazard Mater 161(2–3):1150–1156. https://doi.org/10.1016/j.jhazmat.2008.04.108
Chakraborty P, Sampath S, Mukhopadhyay M, Selvaraj S, Bharat GK, Nizzetto L (2019) Baseline investigation on plasticizers, bisphenol A, polycyclic aromatic hydrocarbons and heavy metals in the surface soil of the informal electronic waste recycling workshops and nearby open dumpsites in Indian metropolitan cities. Environ Pollut 248:1036–1045. https://doi.org/10.1016/j.envpol.2018.11.010
Mojiri A, Zhou JL, Ohashi A, Ozaki N, Kindaichi T (2019) Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci Total Environ 696:133971. https://doi.org/10.1016/j.scitotenv.2019.133971
Cai SS, Syage JA, Hanold KA, Balogh MP (2009) Ultra performance liquid chromatography− atmospheric pressure photoionization-tandem mass spectrometry for high-sensitivity and high-throughput analysis of US environmental protection agency 16 priority pollutants polynuclear aromatic hydrocarbons. Anal Chem 81(6):2123–2128. https://doi.org/10.1021/ac802275e
Yang L, Wu J, Zheng M, Cao Z, Li C, Shi M, Liu G (2020) Non-target screening of organic pollutants and target analysis of halogenated polycyclic aromatic hydrocarbons in the atmosphere around metallurgical plants by high-resolution GC/Q-TOF-MS. Environ Sci Eur 32(1):1–4. https://doi.org/10.1186/s12302-020-00376-9
Luna FM, Oliveira Filho AN, Araújo CC, Azevedo DC, Cavalcante CL Jr (2016) Adsorption of polycyclic aromatic hydrocarbons from heavy naphthenic oil using commercial activated carbons. 1. Fluid-particle studies. Ind Eng Chem Res 55(29):8176–83. https://doi.org/10.1021/acs.iecr.6b01059
Guieysse B, Viklund G, Toes AC, Mattiasson B (2016) Combined UV-biological degradation of PAHs. Chemosphere 55(11):1493–1499. https://doi.org/10.1016/j.chemosphere.2004.01.021
Gan S, Lau EV, Ng HK (2009) Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater 172(2–3):532–549. https://doi.org/10.1016/j.jhazmat.2009.07.118
Bairagi S, Ali SW (2020) Conventional and advanced technologies for wastewater treatment. In: Shahid-ul-Islam, (ed) Environmental Nanotechnology for Water Purification, Wiley, New York 33–56.
Barman SR, Das P, Mukhopadhayay A (2021) Biochar from waste Sterculia foetida and its application as adsorbent for the treatment of PAH compounds: batch and optimization. Fuel 306:121623. https://doi.org/10.1016/j.fuel.2021.121623
Banerjee P, kumarDey T, Sarkar S, Swarnakar S, Mukhopadhyay A, Ghosh S (2016) Treatment of cosmetic effluent in different configurations of ceramic UF membrane based bioreactor: toxicity evaluation of the untreated and treated wastewater using catfish (Heteropneustes fossilis). Chemosphere 146:133–144. https://doi.org/10.1016/j.chemosphere.2015.12.004
Banerjee P, Mukhopadhyay A, Das P (2019) Graphene oxide–nanobentonite composite sieves for enhanced desalination and dye removal. Desalination 451:231–240. https://doi.org/10.1016/j.desal.2017.06.010
Nahar N, Saifuddin S, Sami M (2020) Formulating an Anorganic Membrane Using Clay, Activated Carbon and Micro Zeolite as Filter Media for Peat Water Purification. In: IOP Conference Series: Materials Science and Engineering, IOP Publishing, 854(1) pp 12076. https://iopscience.iop.org/article/10.1088/1757-899X/854/1/012076/pdf
Bahmani E, Koushkbaghi S, Darabi M, ZabihiSahebi A, Askari A, Irani M (2019) Fabrication of novel chitosan-g-PNVCL/ZIF-8 composite nanofibers for adsorption of Cr (VI), As (V) and phenol in a single and ternary systems. Carbohydr Polym 224:115148. https://doi.org/10.1016/j.carbpol.2019.115148
Shariful MI, Sharif SB, Lee JJ, Habiba U, Ang BC, Amalina MA (2017) Adsorption of divalent heavy metal ion by mesoporous-high surface area chitosan/poly (ethylene oxide) nanofibrous membrane. Carbohydr Polym 157:57–64. https://doi.org/10.1016/j.carbpol.2016.09.063
Costa JA, de Jesus RA, da Silva CM, Romão LP (2017) Efficient adsorption of a mixture of polycyclic aromatic hydrocarbons (PAHs) by Si–MCM–41 mesoporous molecular sieve. Powder Technol 308:434–441. https://doi.org/10.1016/j.powtec.2016.12.035
Barman SR, Mukhopadhyay A, Das P (2020) Green synthesis of carbonaceous adsorbents and their application for removal of polyaromatic hydrocarbons (PAHs) from water. In: Sharma SK (ed) Bioremediation, 1st edn. CRC Press Taylor & Francis, Florida, pp 229–258
Herrera-García U, Castillo J, Patiño-Ruiz D, Solano R, Herrera A (2019) Activated carbon from Yam Peels Modified with Fe3O4 for removal of 2, 4-dichlorophenoxyacetic acid in aqueous solution. Water 11(11):2342. https://doi.org/10.3390/w11112342
Xiong Z, Sarmah AK, Padhye LP (2020) Acidic surface functional groups control chemisorption of ammonium onto carbon materials in aqueous media. Sci Total Environ 698:134193. https://doi.org/10.1016/j.scitotenv.2019.134193
Han Q, Wang J, Goodman BA, Xie J, Liu Z (2020) High adsorption of methylene blue by activated carbon prepared from phosphoric acid treated eucalyptus residue. Powder Technol 366:239–248. https://doi.org/10.1016/j.powtec.2020.02.013
Zheng X, Zhou Y, Liu X, Fu X, Peng H, Lv S (2020) Enhanced adsorption capacity of MgO/N-doped active carbon derived from sugarcane bagasse. Bioresour Technol 297:122413. https://doi.org/10.1016/j.biortech.2019.122413
Smith SC, Rodrigues DF (2015) Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon 91:122–143. https://doi.org/10.1016/j.carbon.2015.04.043
He X, Wu Z, Sun Z, Wei X, Wu Z, Ge X, Cravotto G (2018) A novel hybrid of β-cyclodextrin grafted onto activated carbon for rapid adsorption of naphthalene from aqueous solution. J Mol Liq 255:160–167. https://doi.org/10.1016/j.molliq.2018.01.153
Lu F, Astruc D (2020) Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord Chem Rev 408:213180. https://doi.org/10.1016/j.ccr.2020.213180
Barman SR, Roy U, Das P, Mukhopadhayay A (2021) Membrane processes for removal of polyaromatic hydrocarbons from wastewater. In: Sharma SK (ed) Green Chemistry and Water Remediation: Research and Applications, 1st edn. Elsevier, pp 189–207
Solano R, Patiño-Ruiz D, Tejeda-Benitez L, Herrera A (2021) Metal-and metal/oxide-based engineered nanoparticles and nanostructures: a review on the applications, nanotoxicological effects, and risk control strategies. Environ Sci Pollut Res:1–20. https://doi.org/10.1007/s11356-021-12996-6
Degtyareva IA, Ezhkova AM, Yapparov AK, Yapparov IA, Ezhkov VO, Babynin EV, Davletshina AY, Motina TY, Yapparov DA (2016) Production of nano-bentonite and the study of its effect on mutagenesis in bacteria Salmonella typhimurium. Nanotechnol Russ 11(9):663–670. https://doi.org/10.1134/S1995078016050050
Mirzapour P, Kamyab Moghadas B, Tamjidi S, Esmaeili H (2020) Activated carbon/bentonite/Fe3O4 nanocomposite for treatment of wastewater containing Reactive Red 198. Sep Sci Technol 6:1–5. https://doi.org/10.1080/01496395.2020.1843051
Babu AT, Antony R (2019) Clay semiconductor hetero-system of SnO2/bentonite nanocomposites for catalytic degradation of toxic organic wastes. Appl Clay Sci 183:105312. https://doi.org/10.1016/j.clay.2019.105312
Perez JJ, Villanueva ME, Sanchez L, Ollier R, Alvarez V, Copello GJ (2020) Low cost and regenerable composites based on chitin/bentonite for the adsorption potential emerging pollutants. Appl Clay Sci 194:105703. https://doi.org/10.1016/j.clay.2020.105703
Li L, Han D, Wang M, Han Y, Yan H (2020) Molybdenum disulfide–hypercrosslinked polymer composite as an adsorbent for determination of polycyclic aromatic hydrocarbons in environmental water coupled with HPLC–FLD. Microchim Acta 187(4):1–8. https://doi.org/10.1007/s00604-020-4220-0
Solano RA, De León LD, De Ávila G, Herrera AP (2021) Polycyclic aromatic hydrocarbons (PAHs) adsorption from aqueous solution using chitosan beads modified with thiourea, TiO2 and Fe3O4 nanoparticles. Environ Technol Innov 21:101378. https://doi.org/10.1016/j.eti.2021.101378
Muniandy L, Adam F, Mohamed AR, Ng EP (2014) The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Micropor Mesopor Mat 197:316–323. https://doi.org/10.1016/j.micromeso.2014.06.020
Hosseini SM, Amini SH, Khodabakhshi AR, Bagheripour E, Van der Bruggen B (2018) Activated carbon nanoparticles entrapped mixed matrix polyethersulfone based nanofiltration membrane for sulfate and copper removal from water. J Taiwan Inst Chem Eng 82:169–178. https://doi.org/10.1016/j.jtice.2017.11.017
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12(4):214–219. https://doi.org/10.1080/00224065.1980.11980968
Gupta H, Gupta B (2016) Adsorption of polycyclic aromatic hydrocarbons on banana peel activated carbon. Desalin Water Treat 57(20):9498–9509. https://doi.org/10.1080/19443994.2015.1029007
Kumar A, Gupta H (2020) Activated carbon from sawdust for naphthalene removal from contaminated water. Environ Technol Innov 20:101080. https://doi.org/10.1016/j.eti.2020.101080
Zhang B, Yu S, Zhu Y, Shen Y, Gao X, Shi W, Tay JH (2020) Adsorption mechanisms of crude oil onto polytetrafluoroethylene membrane: kinetics and isotherm, and strategies for adsorption fouling control. Sep Purif Technol 235:116212. https://doi.org/10.1016/j.seppur.2019.116212
Awe AA, Opeolu BO, Fatoki OS, Ayanda OS, Jackson VA, Snyman R (2020) Preparation and characterisation of activated carbon from Vitis vinifera leaf litter and its adsorption performance for aqueous phenanthrene. Appl Biol Chem 63(1):1–7. https://doi.org/10.1186/s13765-020-00494-1
Gupta H (2018) Anthracene removal from water onto activated carbon derived from vehicular tyre. Sep Sci Technol 53(4):613–625. https://doi.org/10.1080/01496395.2017.1405038
Qiao K, Tian W, Bai J, Dong J, Zhao J, Gong X, Liu S (2018) Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol Environ Saf 149:80–87. https://doi.org/10.1016/j.ecoenv.2017.11.027
Onwuka KE, Igwe JC, Enenwa NE, Aghalibe CU (2020) A Study of Pyrene Adsorption Behavior onto Oraganoclays in Aqueous Solution. J Ind Pollut Control 6(2): 1–14. http://chemical.journalspub.info/index.php?journal=JPCIP&page=article&op=view&path%5B%5D=1039
Odjadjare EE, Okoh AI (2010) Physicochemical quality of an urban municipal wastewater effluent and its impact on the receiving environment. Environ Monit Assess 170(1):383–394. https://doi.org/10.1007/s10661-009-1240-y
Kim TH, Park C, Kim S (2005) Water recycling from desalination and purification process of reactive dye manufacturing industry by combined membrane filtration. J Clean Prod 13(8):779–786. https://doi.org/10.1016/j.jclepro.2004.02.044
Shannon M A, Bohn P W, Elimelech M, Georgiadis J G, Marinas B J, Mayes A M (2010) Science and technology for water purification in the coming decades. In: Rodgers P (ed) Nanoscience and technology: a collection of reviews from nature Journals, Nature publishing group 337–346. https://doi.org/10.1142/9789814287005_0035
El-Nagar DA, Sary DH (2021) Synthesis and characterization of nano bentonite and its effect on some properties of sandy soils. Soil Tillage Res 208:104872. https://doi.org/10.1016/j.still.2020.104872
Elkony Y, Mansour ES, Elhusseiny A, Hassan H, Ebrahim S (2020) Novel grafted/crosslinked cellulose acetate membrane with N-isopropylacrylamide/N. N-methylenebisacrylamide for water desalination. Sci Rep 10(1):1–3. https://doi.org/10.1038/s41598-020-67008-3
Zhao X, Wu Q, Huang C, Wei H, Wang R, Wang C (2021) Highly efficient separation membrane based on cellulose acetate/chitosan fibrous composite substrate with activated carbon functional adsorption layer. J Chem Technol Biotechnol 96(3):672–679. https://doi.org/10.1002/jctb.6580
Costa NR, Lourenço J, Pereira ZL (2011) Desirability function approach: a review and performance evaluation in adverse conditions. Chemom Intell Lab Syst 107(2):234–244. https://doi.org/10.1016/j.chemolab.2011.04.004
Danish M, Hashim R, Ibrahim MM, Rafatullah M, Ahmad T, Sulaiman O (2011) Characterization of Acacia mangium wood based activated carbons prepared in the presence of basic activating agents. BioResources 6(3):3019–3033
Girods P, Dufour A, Fierro V, Rogaume Y, Rogaume C, Zoulalian A, Celzard A (2009) Activated carbons prepared from wood particleboard wastes: characterisation and phenol adsorption capacities. J Hazard Mater 166(1):491–501. https://doi.org/10.1016/j.jhazmat.2008.11.047
Dada AO, Adekola FA, Odebunmi EO (2017) Kinetics, mechanism, isotherm and thermodynamic studies of liquid phase adsorption of Pb2+ onto wood activated carbon supported zerovalent iron (WAC-ZVI) nanocomposite. Cogent Chem 3(1):1351653. https://doi.org/10.1080/23312009.2017.1351653
Abukhadra MR, Adlii A, Bakry BM (2019) Green fabrication of bentonite/chitosan@ cobalt oxide composite (BE/CH@ Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int J Biol Macromol 126:402–413. https://doi.org/10.1016/j.ijbiomac.2018.12.225
Bouyahmed F, Cai M, Reinert L, Duclaux L, Dey RK, Youcef HB, Lahcini M, Muller F, Delpeux-Ouldriane S (2018) A wide adsorption range hybrid material based on chitosan, activated carbon and montmorillonite for water treatment C 4(2):35. https://doi.org/10.3390/c4020035
Lai DS, Osman AF, Adnan SA, Ibrahim I, Alrashdi AA, Ahmad Salimi MN, Ul-Hamid A (2021) On the use of OPEFB-derived microcrystalline cellulose and nano-bentonite for development of thermoplastic starch hybrid bio-composites with improved performance. Polymers 13(6):897. https://doi.org/10.3390/polym13060897
Shrestha D, Gyawali G, Rajbhandari A (2018) Preparation and characterization of activated carbon from waste sawdust from saw mill. JIST 22(2):103–108. https://doi.org/10.3126/jist.v22i2.19600
Idohou EA, Fatombi JK, Osseni SA, Agani I, Neumeyer D, Verelst M, Mauricot R, Aminou T (2020) Preparation of activated carbon/chitosan/Carica papaya seeds composite for efficient adsorption of cationic dye from aqueous solution. Surf Interfaces 21:100741. https://doi.org/10.1016/j.surfin.2020.100741
Fatombi JK, Idohou EA, Osseni SA, Agani I, Neumeyer D, Verelst M, Mauricot R, Aminou T (2019) Adsorption of indigo carmine from aqueous solution by chitosan and chitosan/activated carbon composite: kinetics, isotherms and thermodynamics studies. Fibers Polym 20(9):1820–1832. https://doi.org/10.1007/s12221-019-1107-y
Mohammada SG, Abulyaziedb DE, Ahmedb SM (2019) Application of polyaniline/activated carbon nanocomposites derived from different agriculture wastes for the removal of Pb (II) from aqueous media. Desalination Water Treat 2019https://doi.org/10.5004/dwt.2019.24694
Motawie AM, Madany MM, El-Dakrory AZ, Osman HM, Ismail EA, Badr MM, El-Komy DA, Abulyazied DE (2014) Physico-chemical characteristics of nano-organo bentonite prepared using different organo-modifiers. Egypt J Pet 23(3):331–338. https://doi.org/10.1016/j.ejpe.2014.08.009
Fernandes C, Catrinescu C, Castilho P, Russo PA, Carrott MR, Breen C (2007) Catalytic conversion of limonene over acid activated Serra de Dentro (SD) bentonite. Appl Catal A-Gen 318:108–120. https://doi.org/10.1016/j.apcata.2006.10.048
Taha AA, Ahmed AM, Abdel Rahman HH, Abouzeid FM, Abdel Maksoud MO (2017) Removal of nickel ions by adsorption on nano-bentonite: equilibrium, kinetics, and thermodynamics. J Dispers Sci Technol 38(5):757–767. https://doi.org/10.1080/01932691.2016.1194211
Lua AC, Yang T (2004) Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J Colloid Interface Sci 274(2):594–601. https://doi.org/10.1016/j.jcis.2003.10.001
Alshuiael SM, Al-Ghouti MA (2020) Multivariate analysis for FTIR in understanding treatment of used cooking oil using activated carbon prepared from olive stone. PLoS One 15(5):e0232997. https://doi.org/10.1371/journal.pone.0232997
Li Z, Hanafy H, Zhang L, Sellaoui L, Netto MS, Oliveira ML, Seliem MK, Dotto GL, Bonilla-Petriciolet A, Li Q (2020) Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem Eng J 388:124263. https://doi.org/10.1016/j.cej.2020.124263
Ali R, Aslam Z, Shawabkeh RA, Asghar A, Hussein IA (2020) BET, FTIR, and RAMAN characterizations of activated carbon from waste oil fly ash. Turk J Chem 44(2):279–295. https://doi.org/10.3906/kim-1909-20
Tang C, Hu D, Cao Q, Yan W, Xing B (2017) Silver nanoparticles-loaded activated carbon fibers using chitosan as binding agent: preparation, mechanism, and their antibacterial activity. Appl Surf Sci 394:457–465. https://doi.org/10.1016/j.apsusc.2016.10.095
Ouaissa YA, Djbrouni A, Chabani S, Chabani M, Amrane A, Bensmaili A (2021) Microwave assisted preparation of onion skin activated carbon: application for removal of tetracycline. AJCE 1(01):16–23. https://doi.org/10.5281/zenodo.4670571
Sellaoui L, Soetaredjo FE, Ismadji S, Bonilla-Petriciolet A, Belver C, Bedia J, Lamine AB, Erto A (2018) Insights on the statistical physics modeling of the adsorption of Cd2+ and Pb2+ ions on bentonite-chitosan composite in single and binary systems. Chem Eng J 354:569–576. https://doi.org/10.1016/j.cej.2018.08.073
Topuz F, Holtzl T, Szekely G (2021) Scavenging organic micropollutants from water with nanofibrous hypercrosslinked cyclodextrin membranes derived from green resources. Chem Eng J 419:129443. https://doi.org/10.1016/j.cej.2021.129443
Lv Y, Ma J, Liu K, Jiang Y, Yang G, Liu Y, Lin C, Ye X, Shi Y, Liu M, Chen L (2021) Rapid elimination of trace bisphenol pollutants with porous β-cyclodextrin modified cellulose nanofibrous membrane in water: adsorption behavior and mechanism. J Haz Mater 403:123666. https://doi.org/10.1016/j.jhazmat.2020.123666
Li S, Luo J, Hang X, Zhao S, Wan Y (2019) Removal of polycyclic aromatic hydrocarbons by nanofiltration membranes: rejection and fouling mechanisms. J Membr Sci 582:264–273. https://doi.org/10.1016/j.memsci.2019.04.008
Du X, Shi Y, Jegatheesan V, Haq IU (2020) A review on the mechanism, impacts and control methods of membrane fouling in MBR system. Membr 10(2):24. https://doi.org/10.3390/membranes10020024
Esmaeili A, Beni AA (2014) A novel fixed-bed reactor design incorporating an electrospun PVA/chitosan nanofiber membrane. J Hazard Mater 280:788–796. https://doi.org/10.1016/j.jhazmat.2014.08.048
Benhouria A, Islam MA, Zaghouane-Boudiaf H, Boutahala M, Hameed BH (2015) Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chem Eng J 270:621–630. https://doi.org/10.1016/j.cej.2015.02.030
Li J, Jiang B, Liu Y, Qiu C, Hu J, Qian G, Guo W, Ngo HH (2017) Preparation and adsorption properties of magnetic chitosan composite adsorbent for Cu2+ removal. J Clean Prod 158:51–58. https://doi.org/10.1016/j.jclepro.2017.04.156
Elwakeel KZ, Aly MH, El-Howety MA, El-Fadaly E, Al-Said A (2018) Synthesis of chitosan@ activated carbon beads with abundant amino groups for capture of Cu (II) and Cd (II) from aqueous solutions. J Polym Environ 26(9):3590–3602. https://doi.org/10.1007/s10924-018-1243-2
Malakootian M, Nasiri A, Mahdizadeh H (2018) Preparation of CoFe2O4/activated carbon@ chitosan as a new magnetic nanobiocomposite for adsorption of ciprofloxacin in aqueous solutions. Water Sci Technol 78(10):2158–2170. https://doi.org/10.2166/wst.2018.494
Mahmoud ME, El-Ghanam AM, Mohamed RH, Saad SR (2020) Enhanced adsorption of Levofloxacin and Ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Mater Sci Eng C 108:110199. https://doi.org/10.1016/j.msec.2019.110199
Xu X, Cheng Y, Wu X, Fan P, Song R (2020) La (III)-bentonite/chitosan composite: a new type adsorbent for rapid removal of phosphate from water bodies. Appl Clay Sci 190:105547. https://doi.org/10.1016/j.clay.2020.105547
Acknowledgements
The authors acknowledge all the members of the Department of Environmental Science, University of Calcutta and the Department of Chemical Engineering, Jadavpur University for their support and cooperation throughout this study. The authors also acknowledge Center for Research in Nanoscience and Nanotechnology and Department of Polymer Science, University of Calcutta, for the electron microscopy, EDS and FTIR facilities. The Authors also acknowledges Indian Institute of Science Education and Research Kolkata for providing the BET surface area measurement facility.
Funding
This research was funded by the department.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Green fabrication of a novel chitosan-linked-activated carbon-nano-bentonite composite membrane for PAH removal from aqueous solutions.
• Novel membrane revealed a high-specific surface area and good reusability potential.
• AC-NB-C membrane was efficient in removing acenaphthene and naphthalene individually as well as in mixtures.
• Process optimization yielded high removal of mixed PAHs (≈99.3%).
• Alkali wash provided excellent flux recovery for AC-NB-C membranes.
• AC-NB-C membrane was eco-friendly, inexpensive, and demonstrated efficient anti fouling properties.
Rights and permissions
About this article
Cite this article
Barman, S.R., Banerjee, P., Mukhopadhayay, A. et al. Biopolymer linked activated carbon-nano-bentonite composite membrane for efficient elimination of PAH mixture from aqueous solutions. Biomass Conv. Bioref. 14, 359–373 (2024). https://doi.org/10.1007/s13399-021-02223-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02223-0